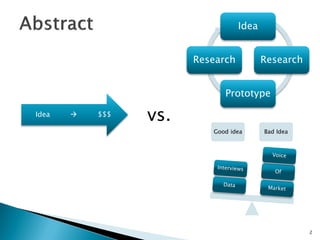
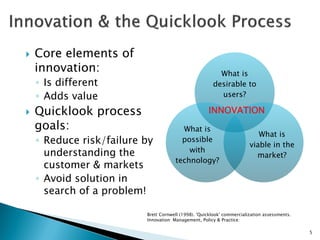
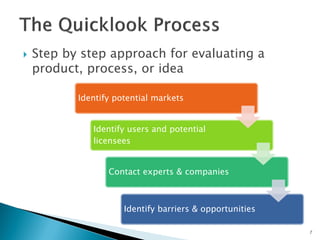
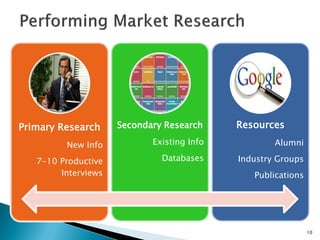
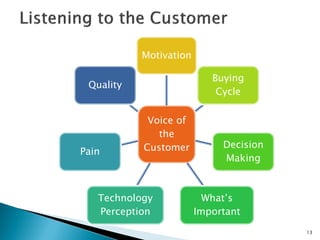
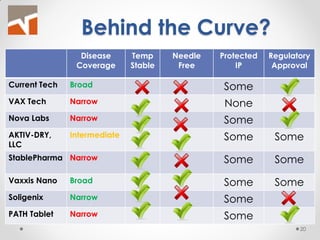
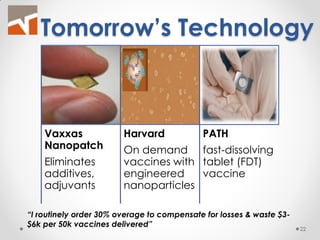
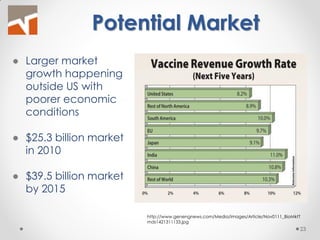
The document discusses the challenges of innovation and product commercialization, highlighting high failure rates for new products, especially in startups. It introduces a 'Quicklook' process designed to assess and evaluate market opportunities and technology viability through structured research and customer engagement. Additionally, it outlines the development of a new dry vaccine technology aimed at improving immunization coverage in resource-limited settings.