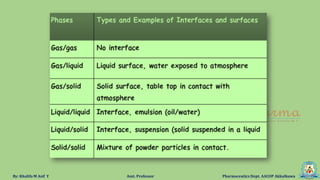
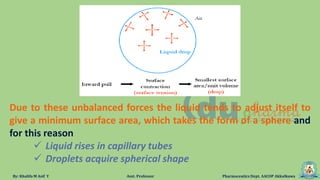
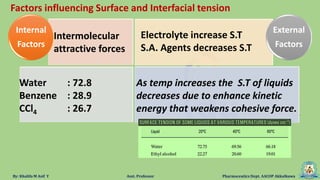
The document discusses surface tension, defining it as the force acting along the surface of a liquid. It explains how surface molecules experience a net inward pull due to attractive forces, resulting in surface tension, which behaves like a stretched elastic membrane. Additionally, it highlights the significance of surface tension in various phenomena such as the formation of droplets and the rise of liquids in capillary tubes.