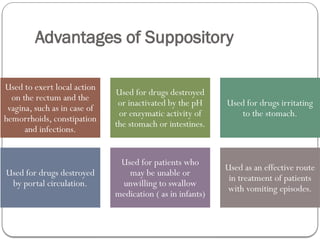
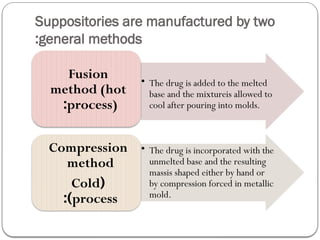
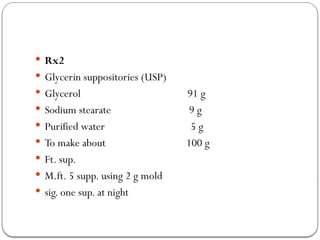
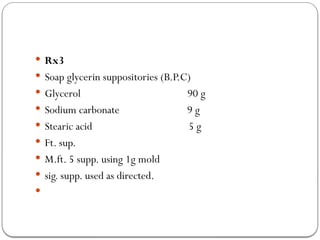
The document outlines the formulation and preparation of suppositories, detailing their types, advantages, and methods of manufacture. It covers both fatty and water-soluble bases, explaining their properties and the processes involved in creating suppositories with various APIs. Additionally, the text includes practical examples of dosage calculations and experimental procedures for different suppository formulations.