The document provides information on the microstructure and macrostructure of various textile fibres, including:
- Cellulose fibre structure and the polymer system and microstructure of cotton fibres.
- Protein fibre structures such as wool, silk, and their chemical composition and microstructures.
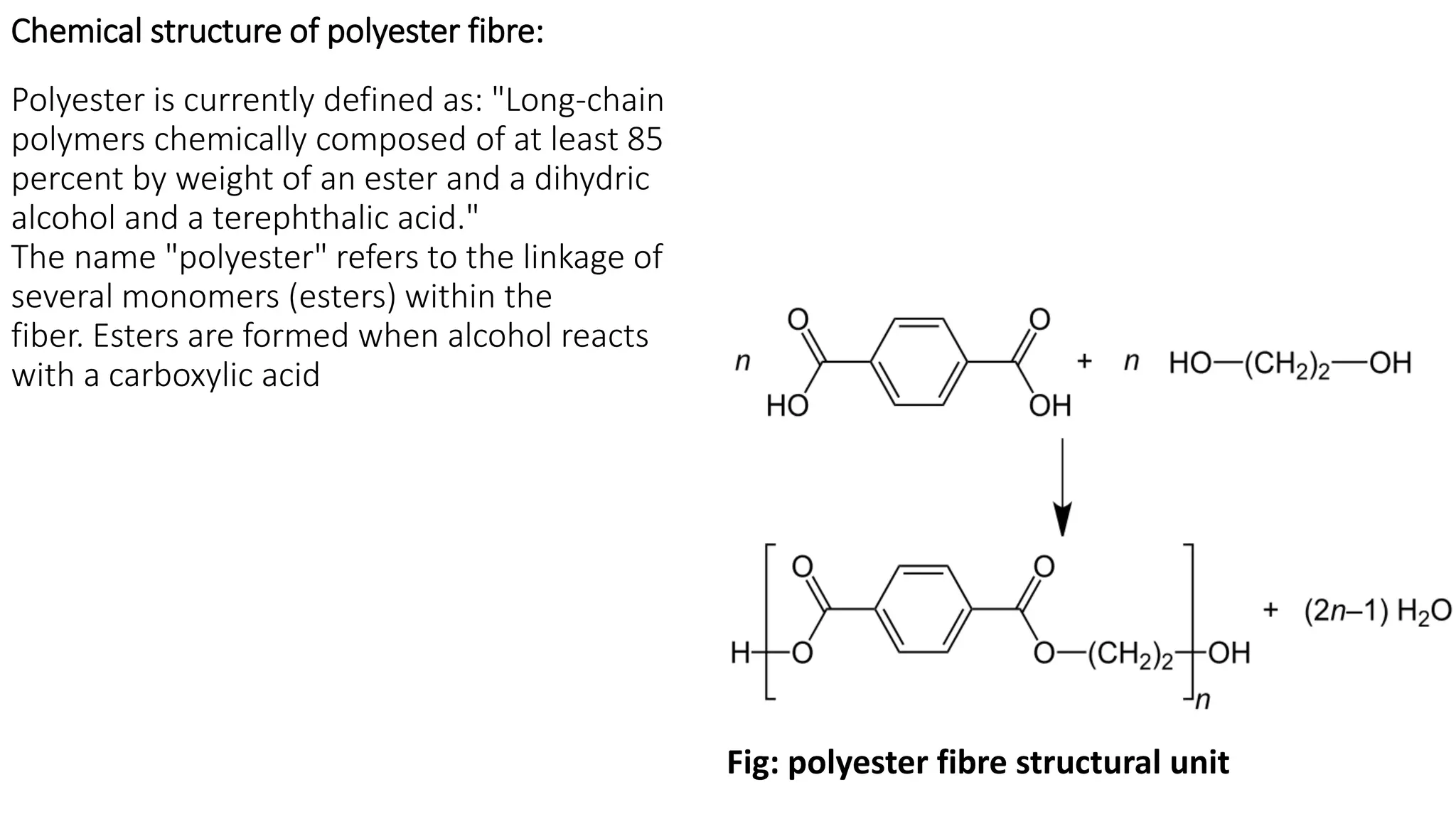
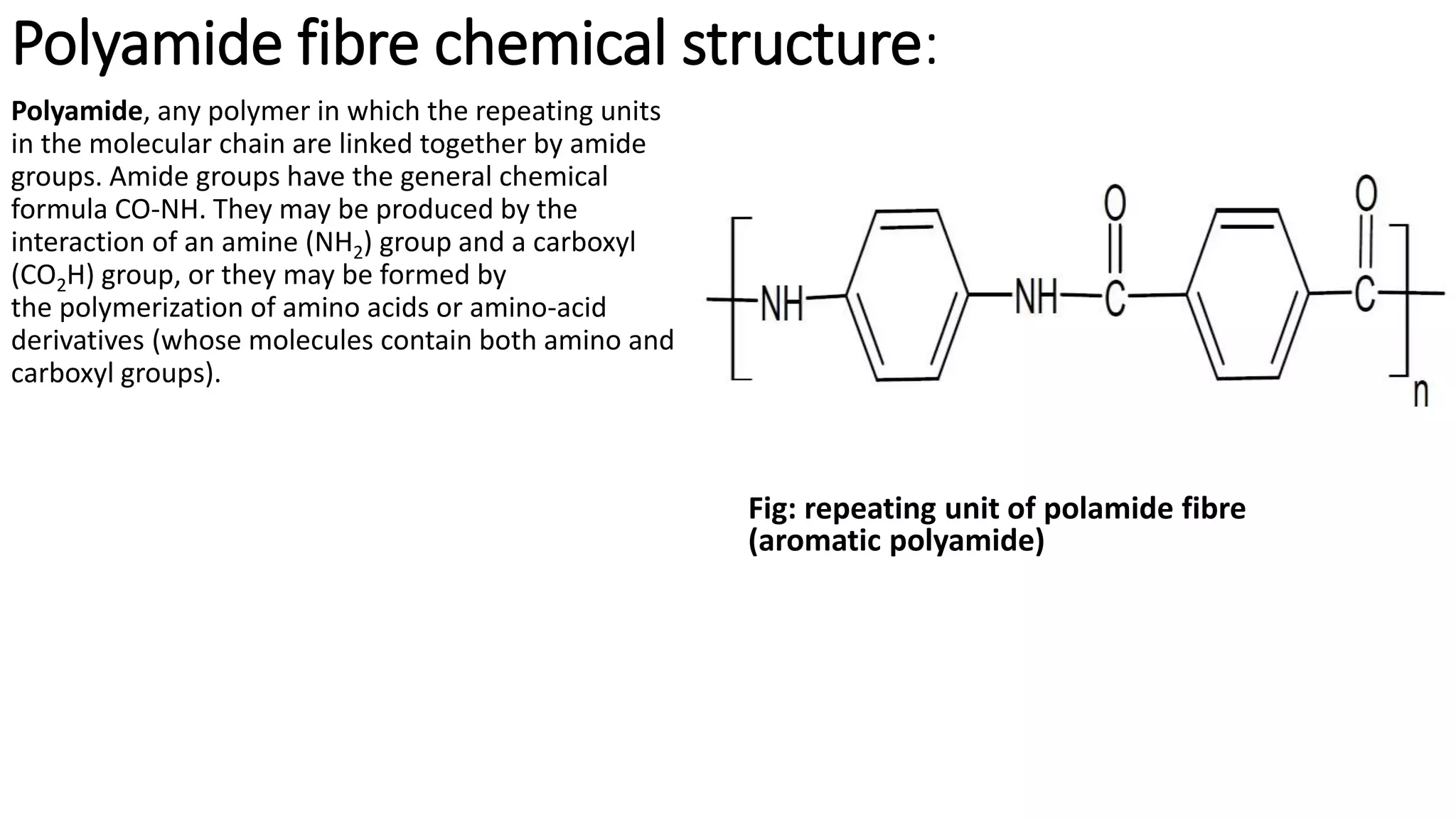
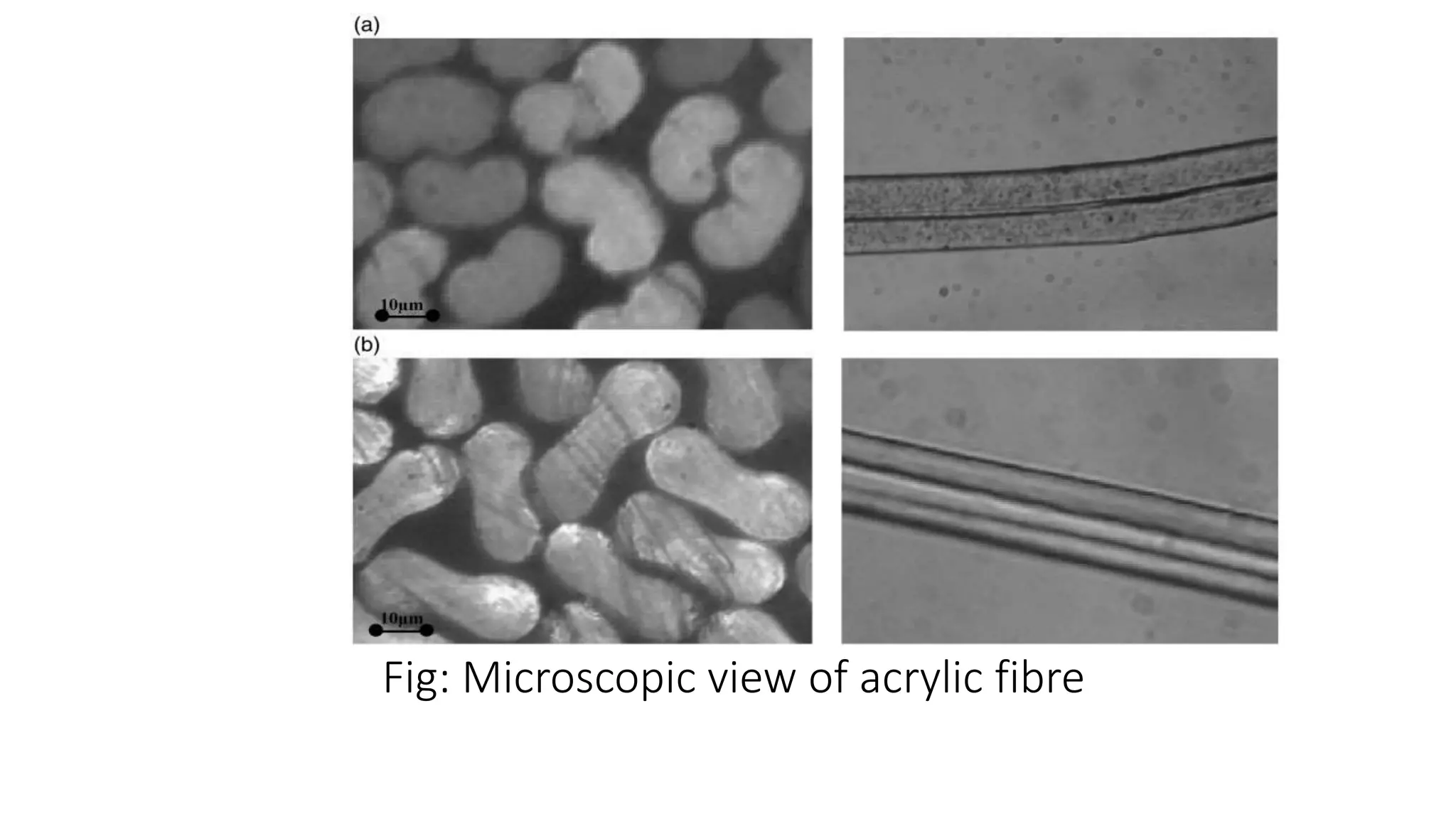
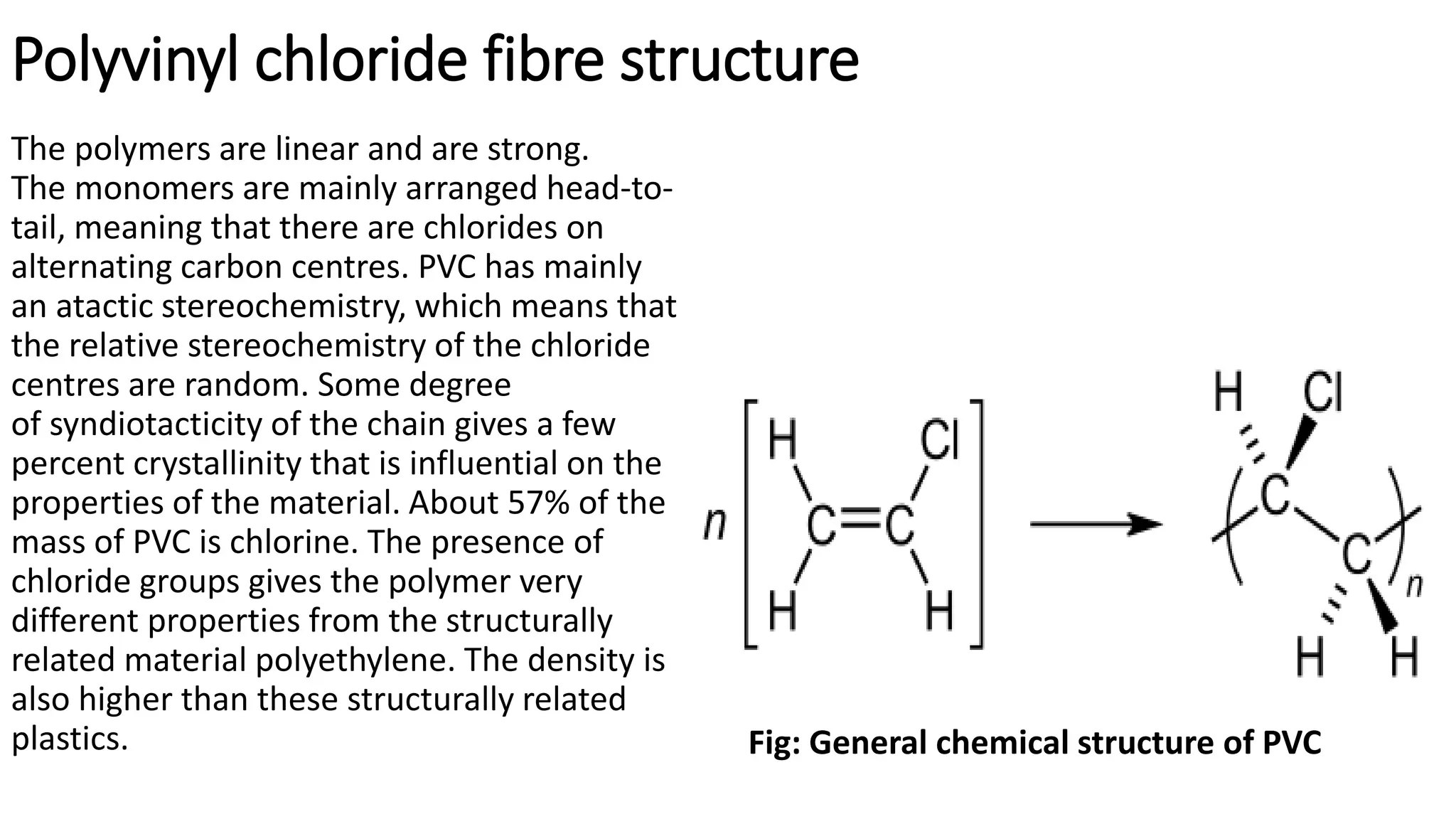
- Synthetic fibre structures like polyester, polyamide, acrylic, and polyvinyl chloride. Their chemical structures and bonding are described.
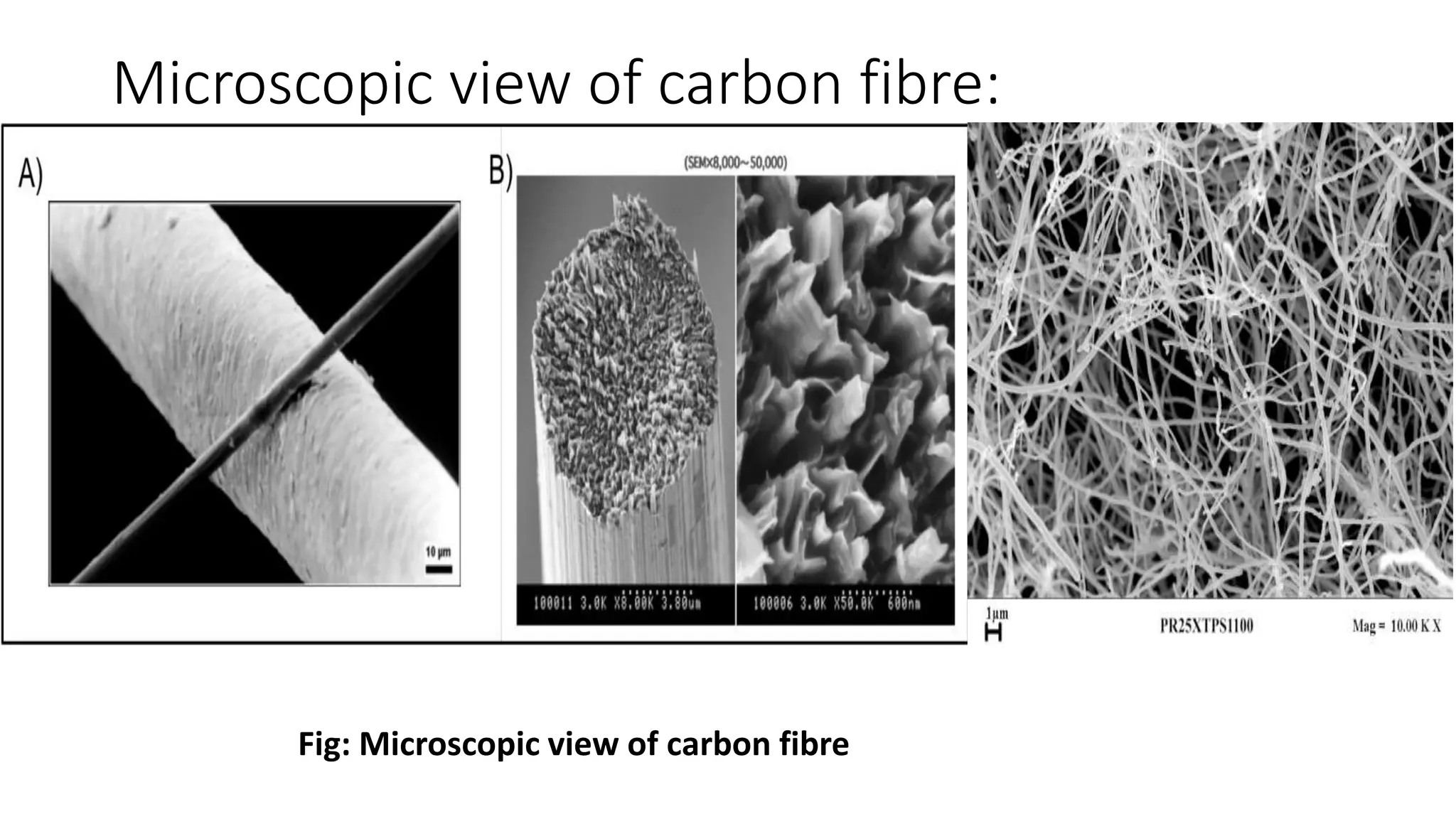
- Microscopic views of fibres are provided along with descriptions of fibre dimensions, cross-sections, and crystalline structures.