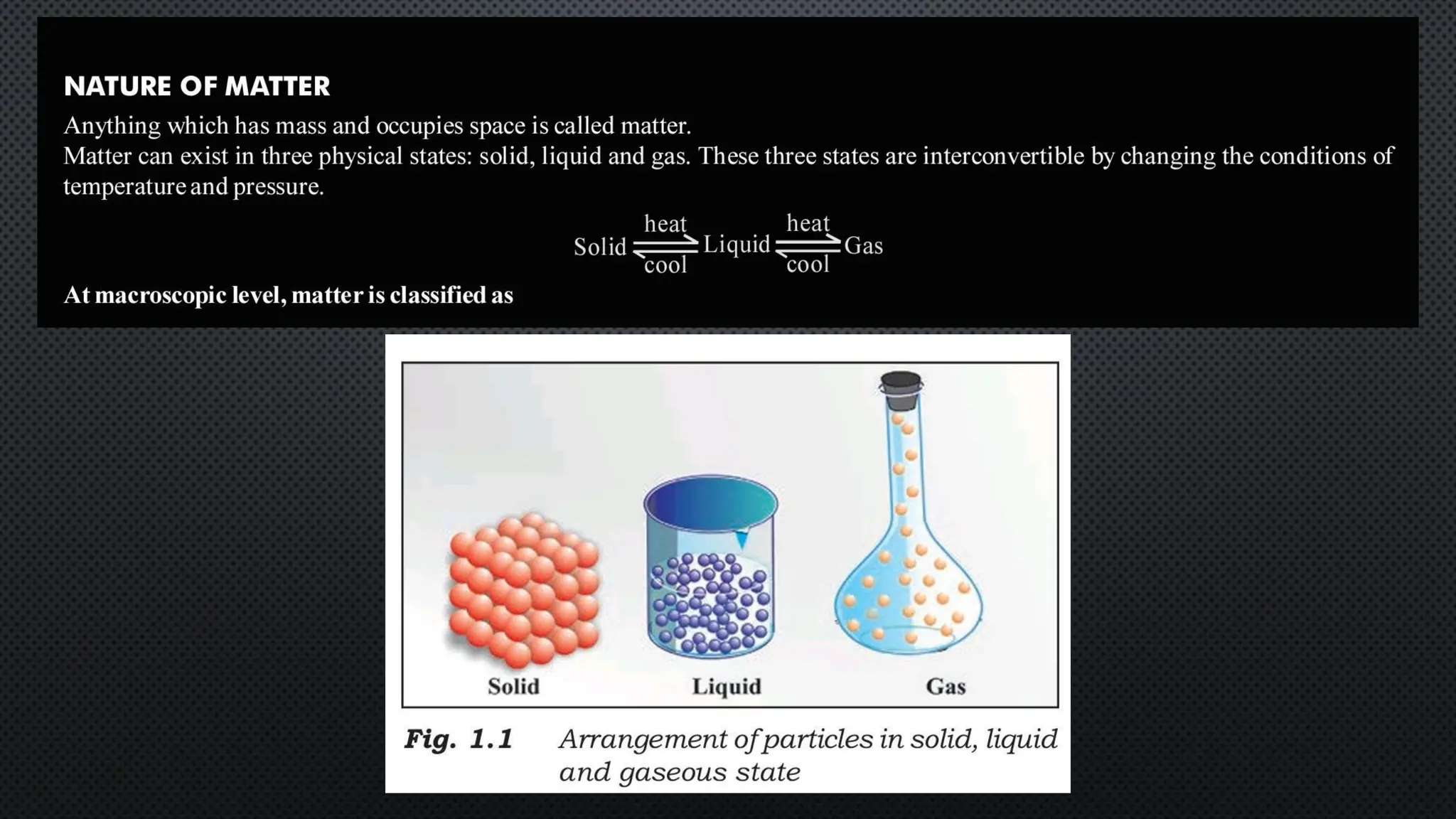
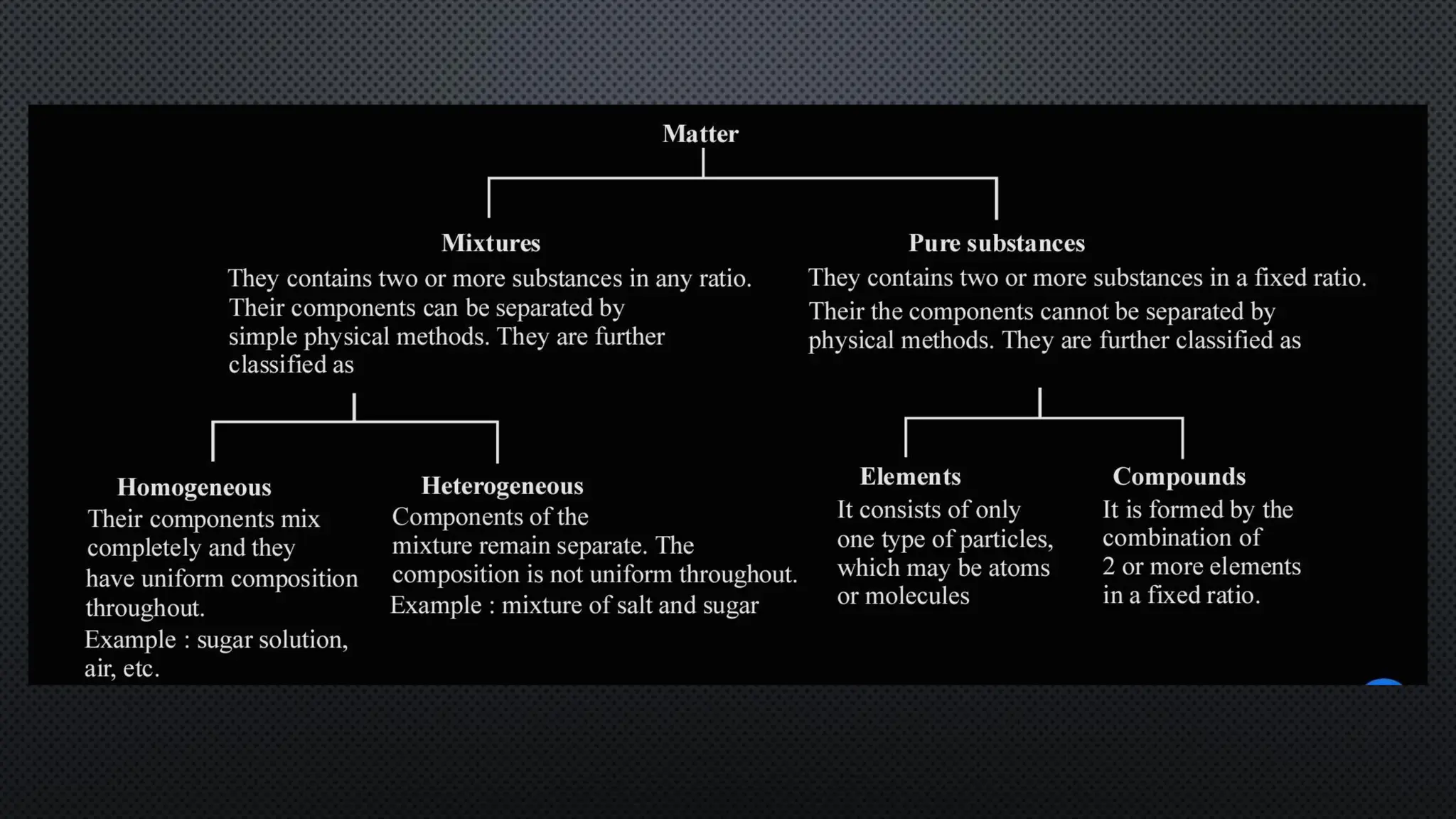
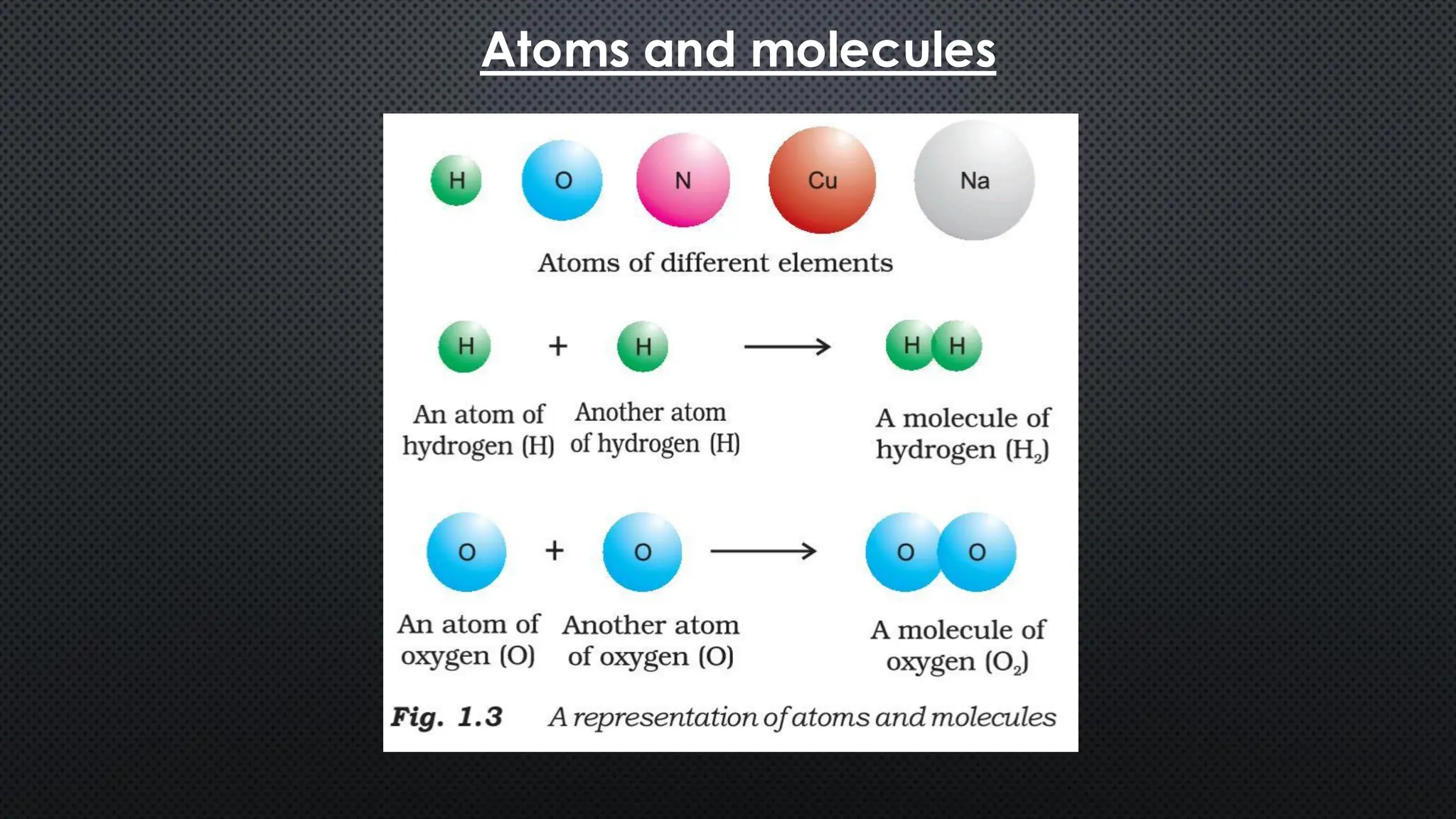
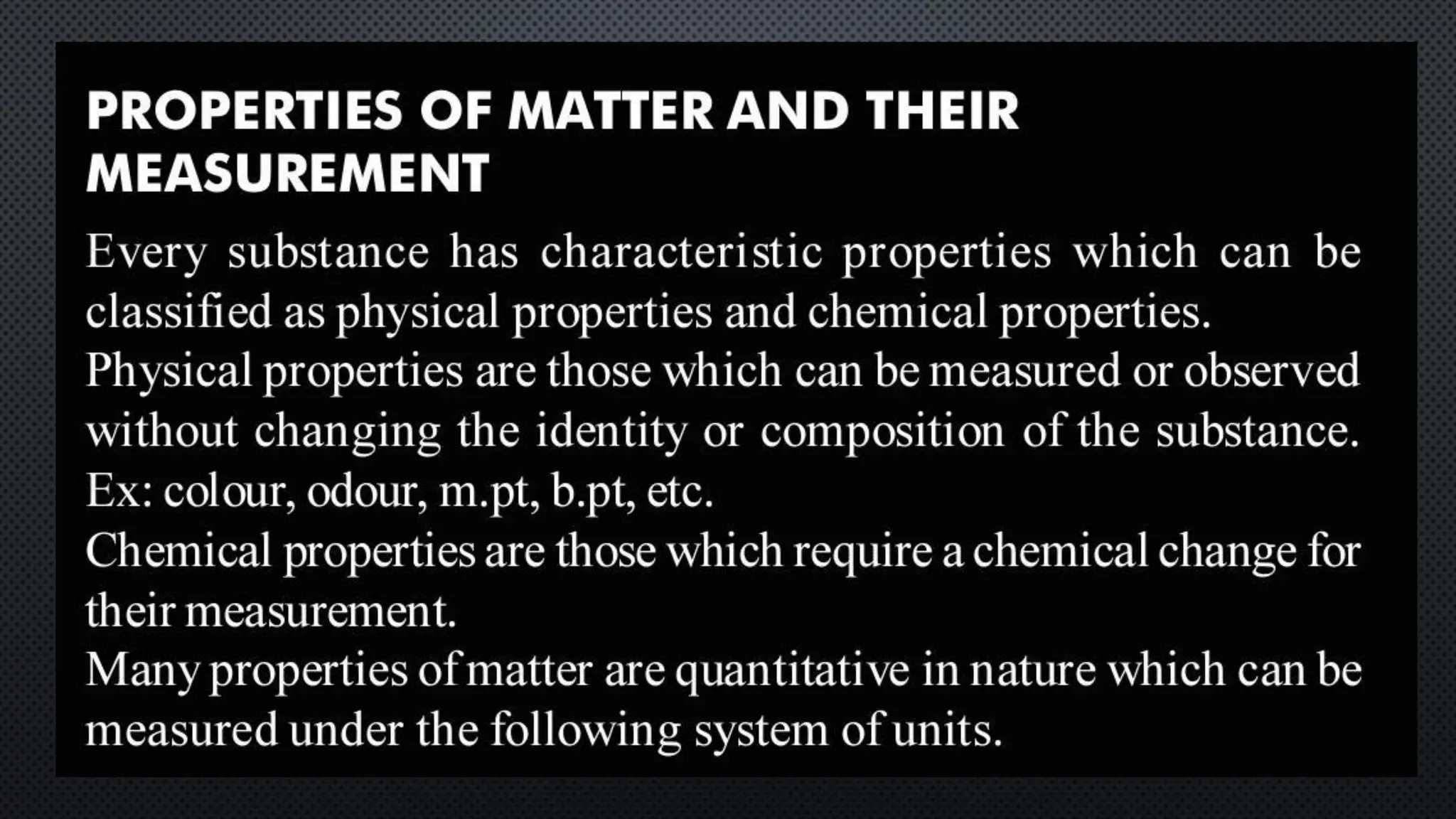
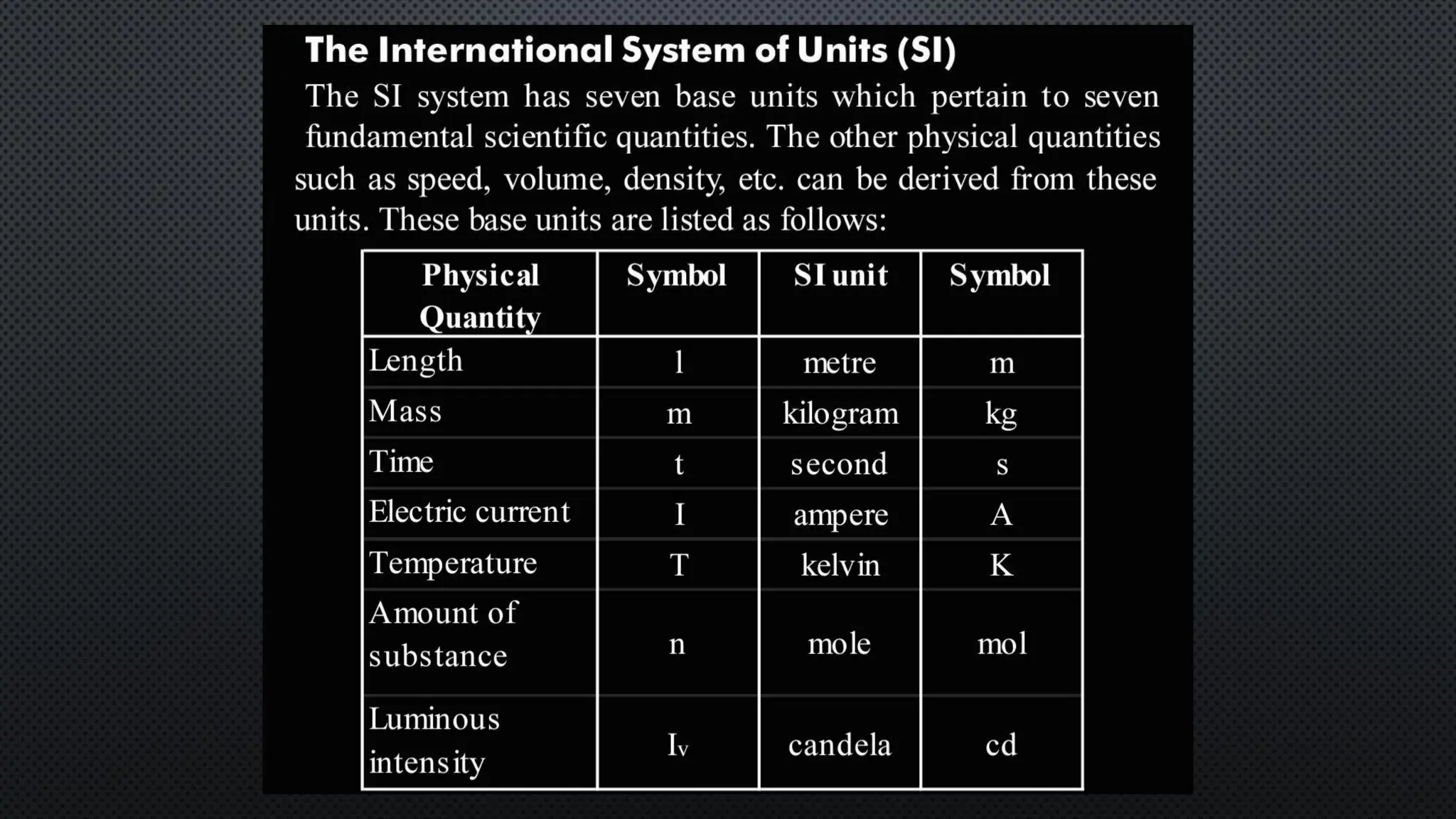
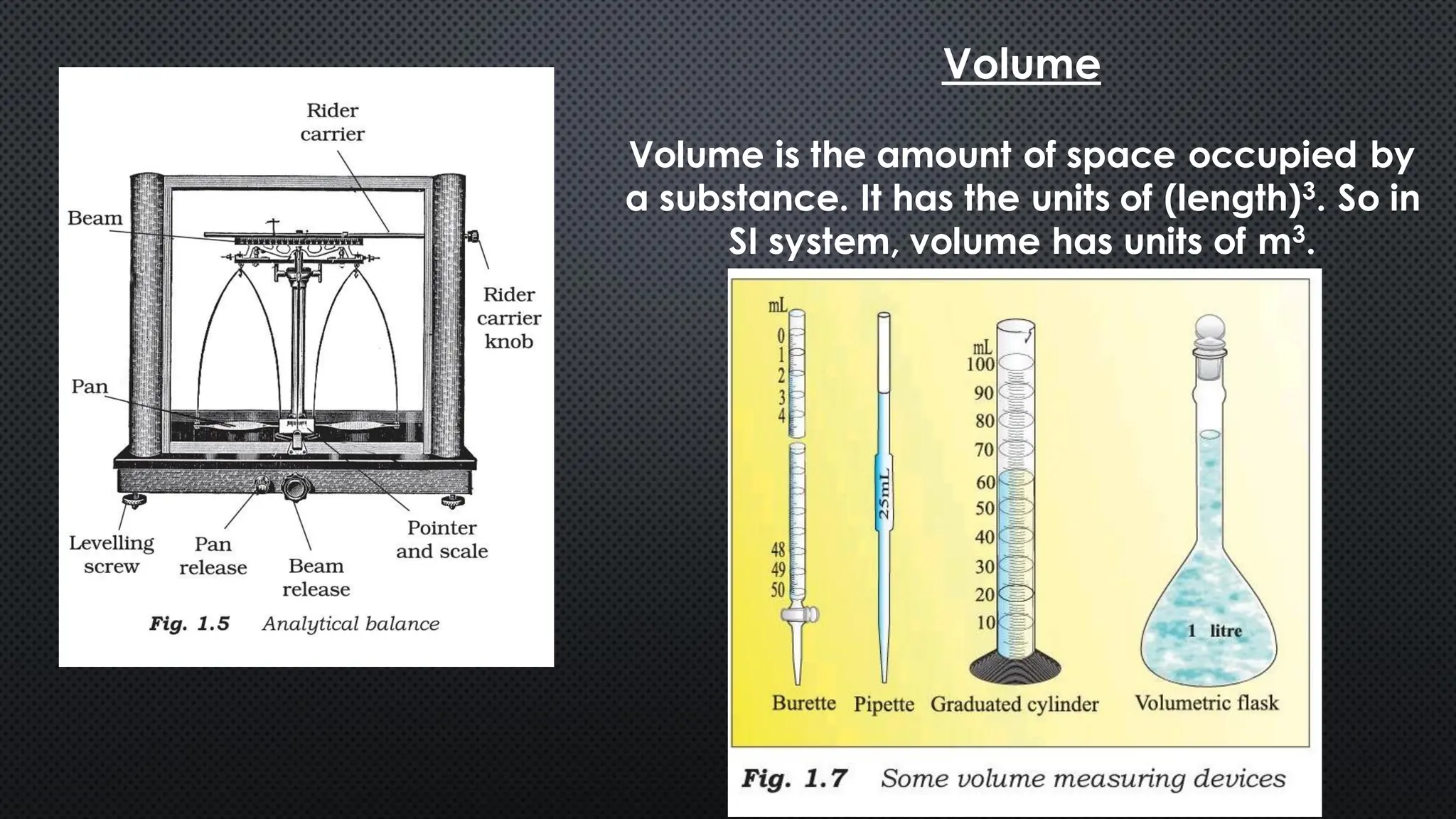
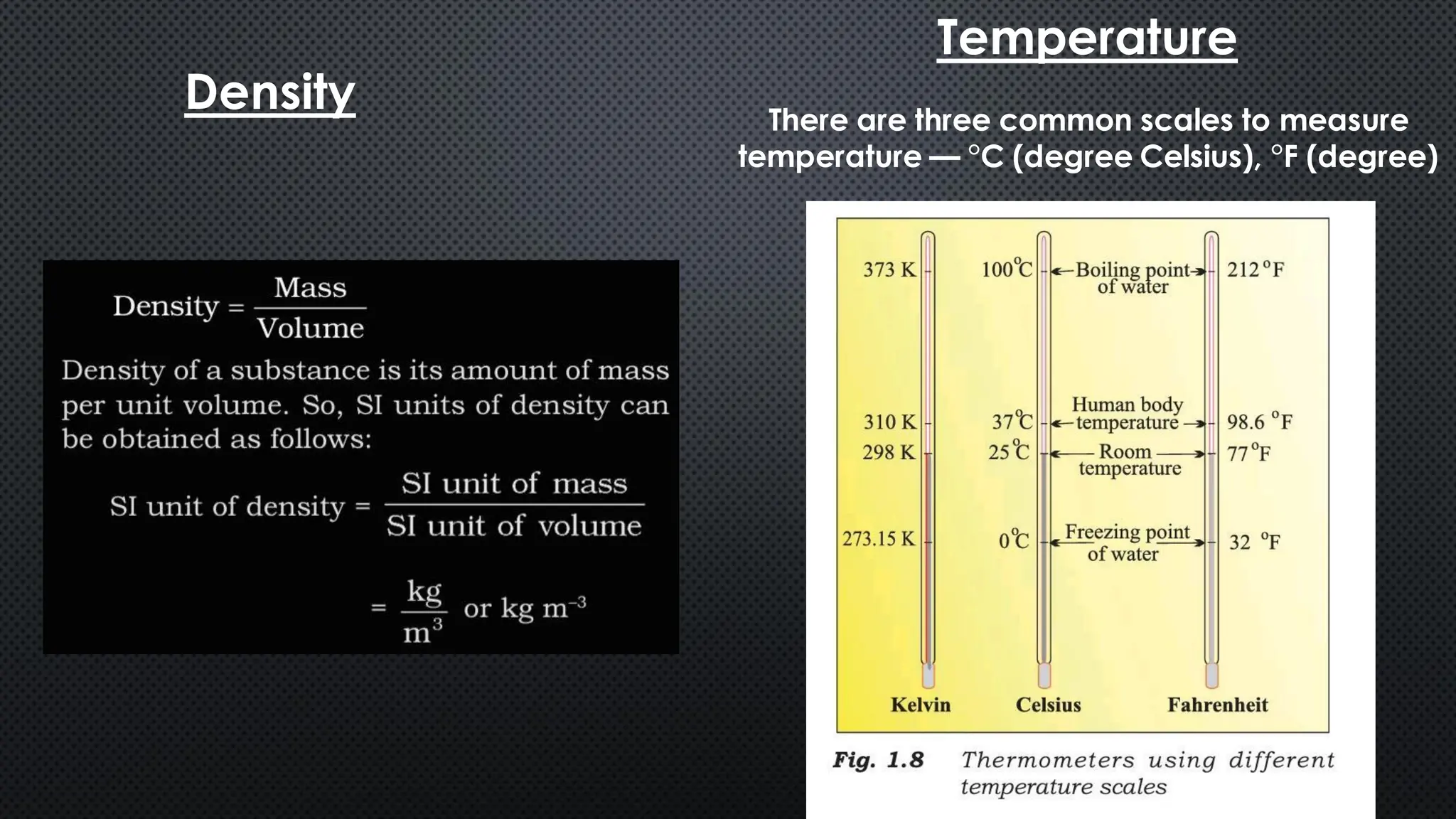
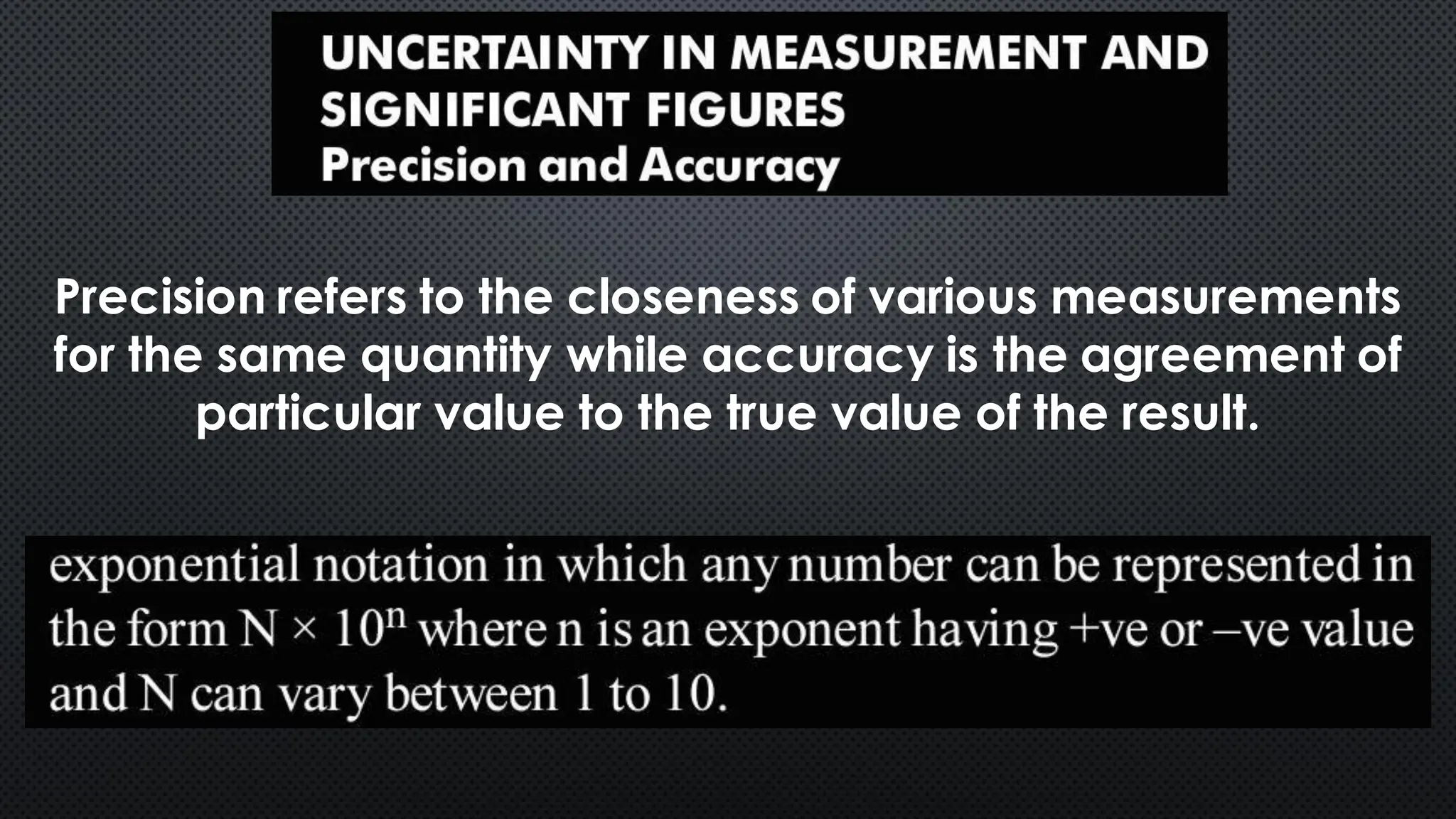
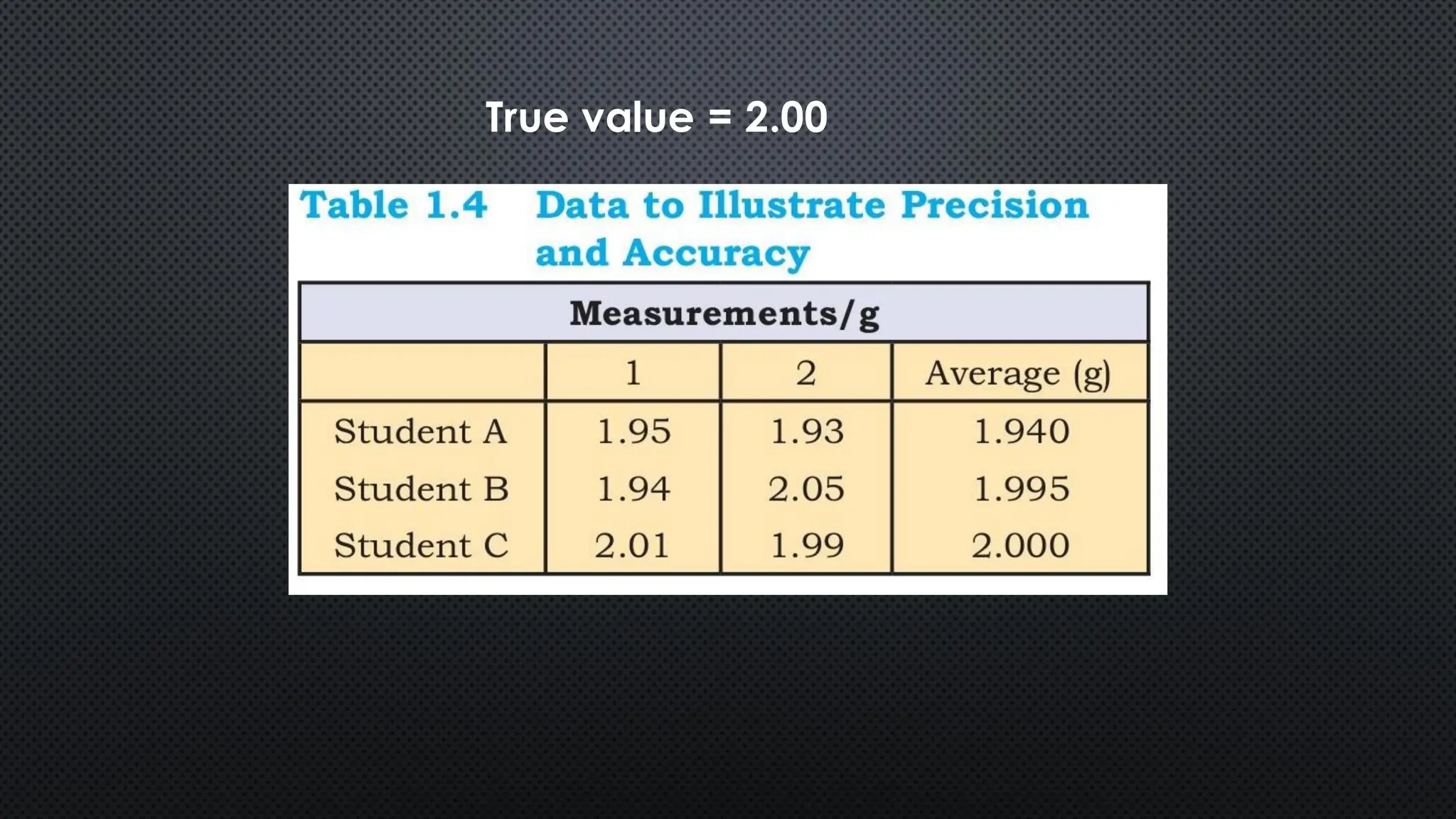
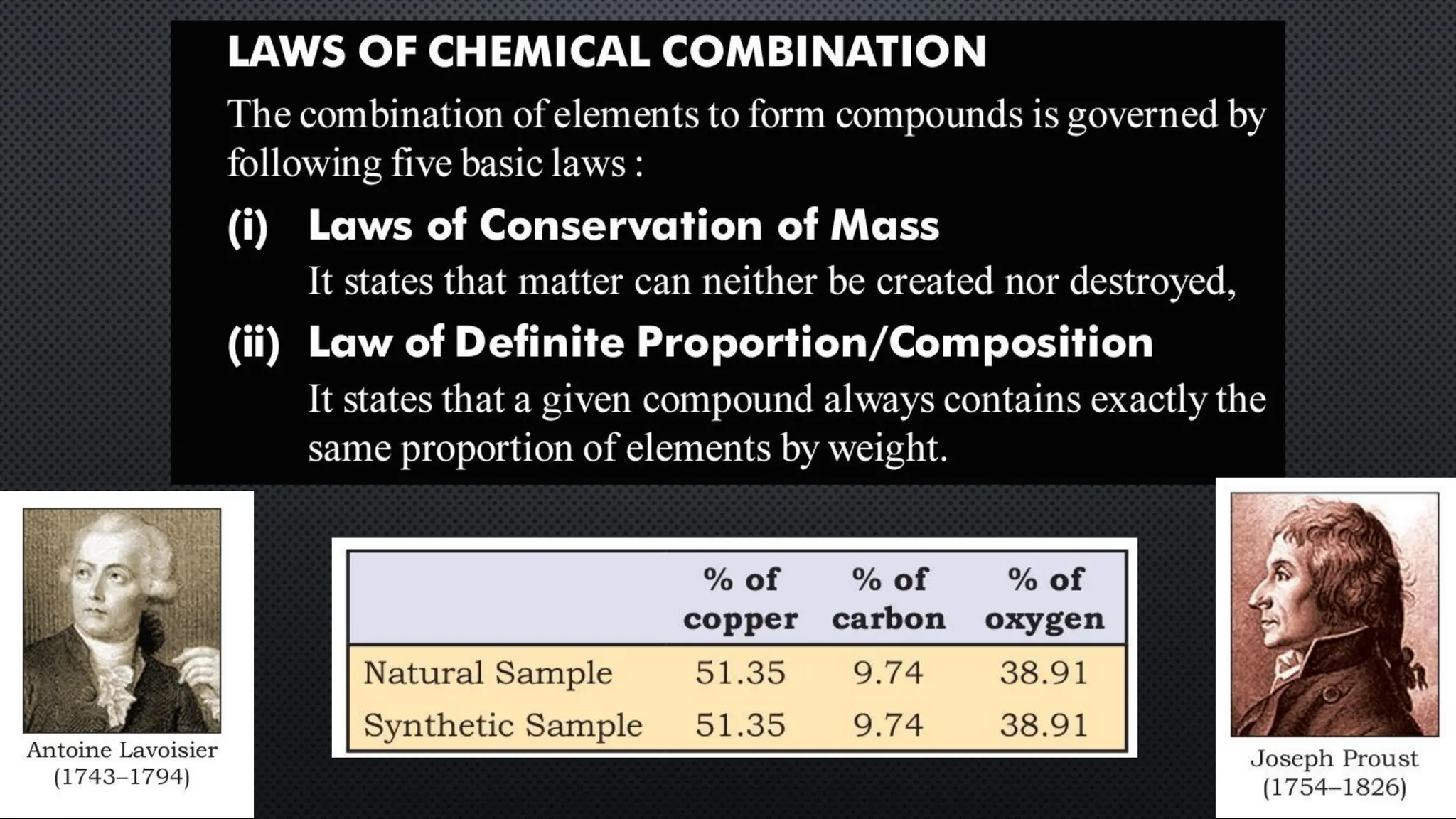
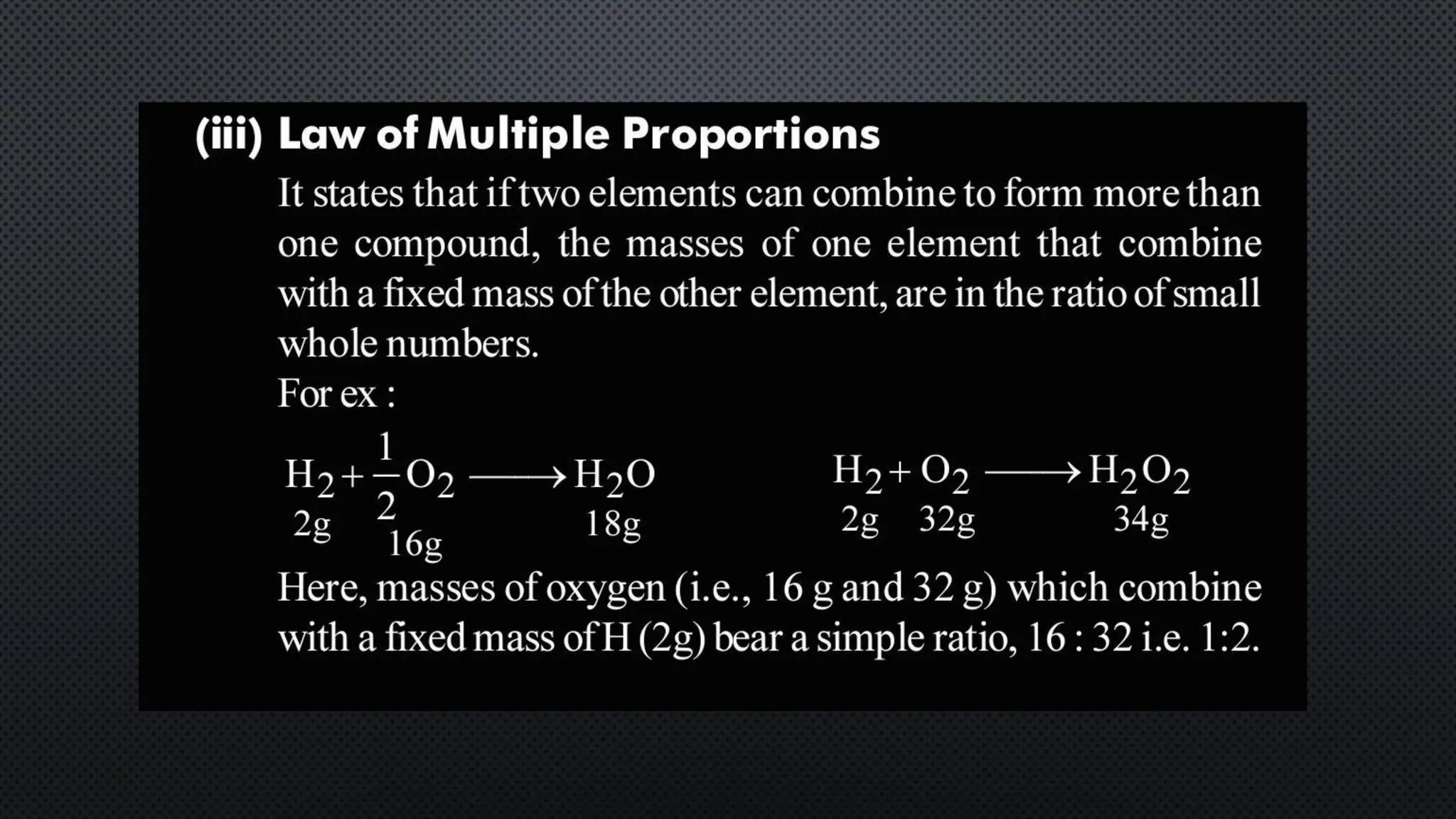
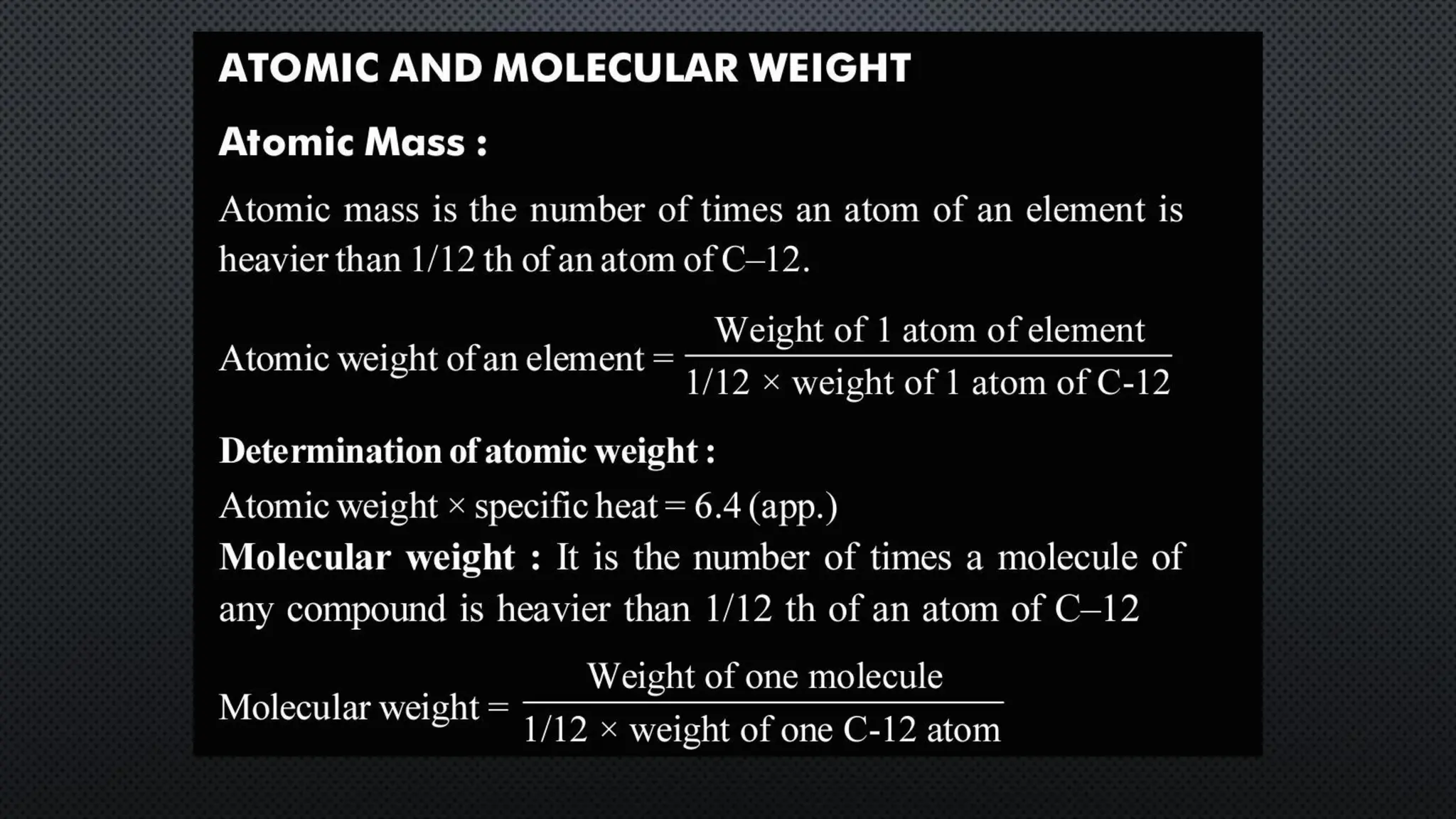
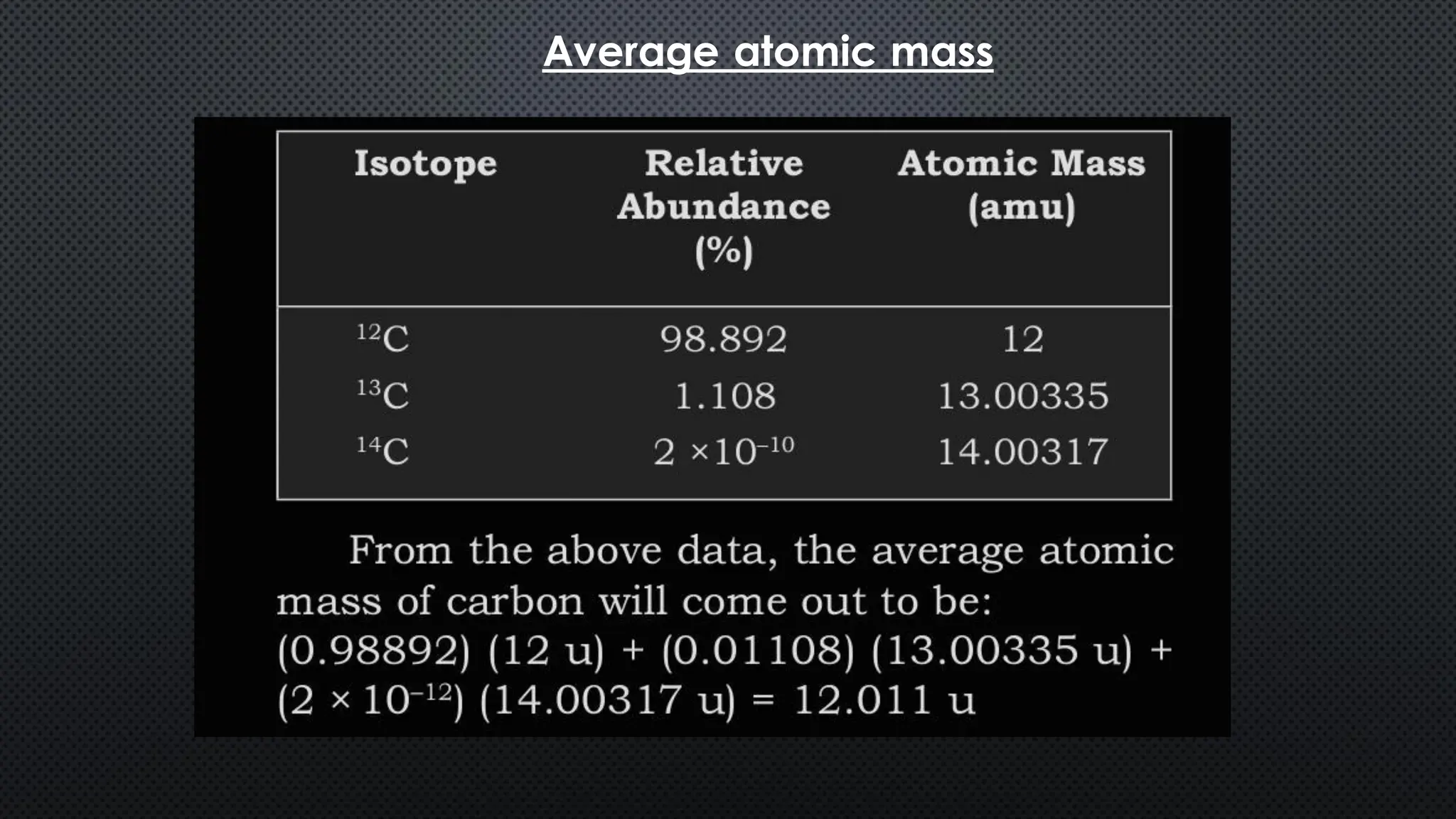
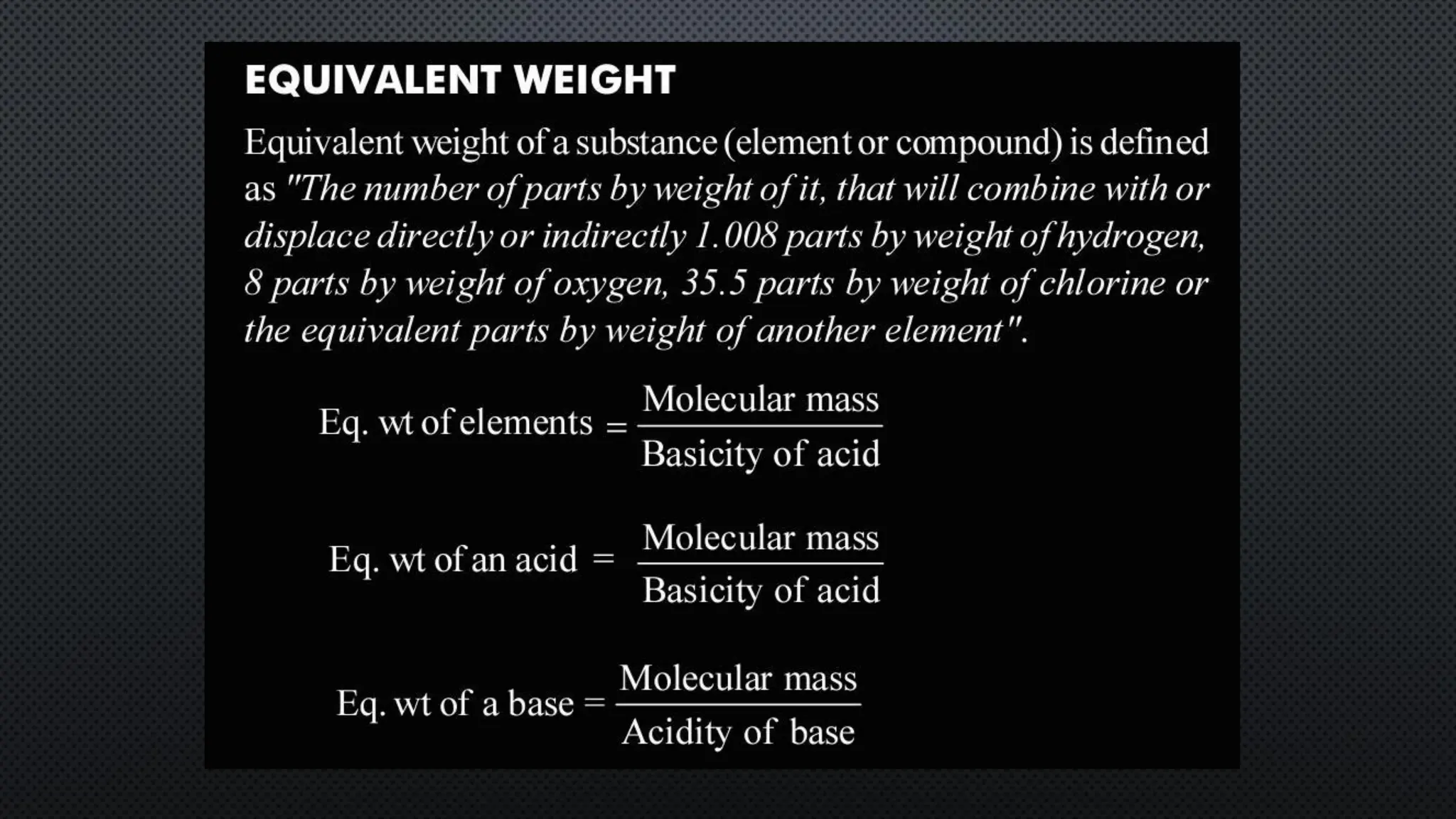
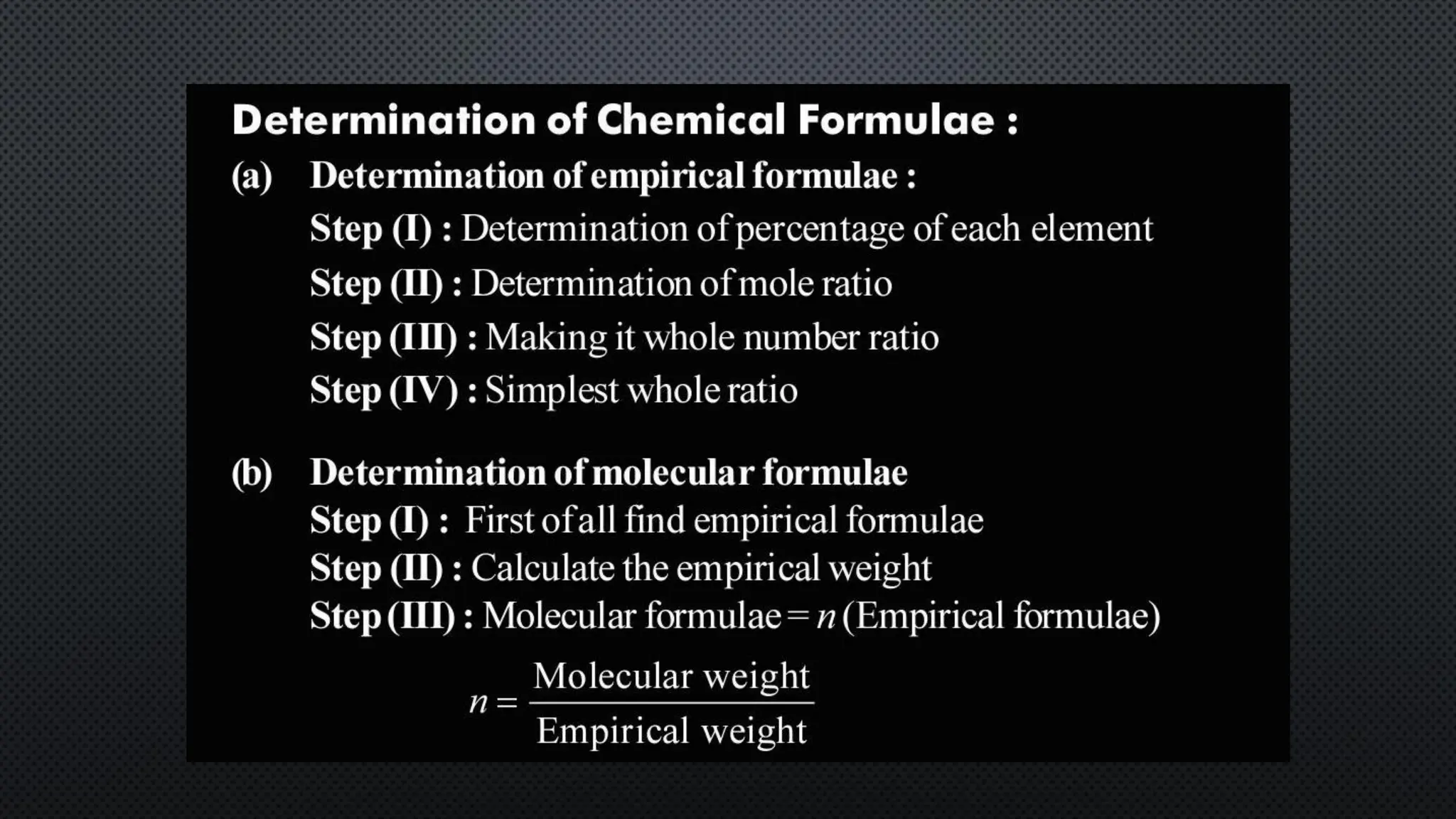
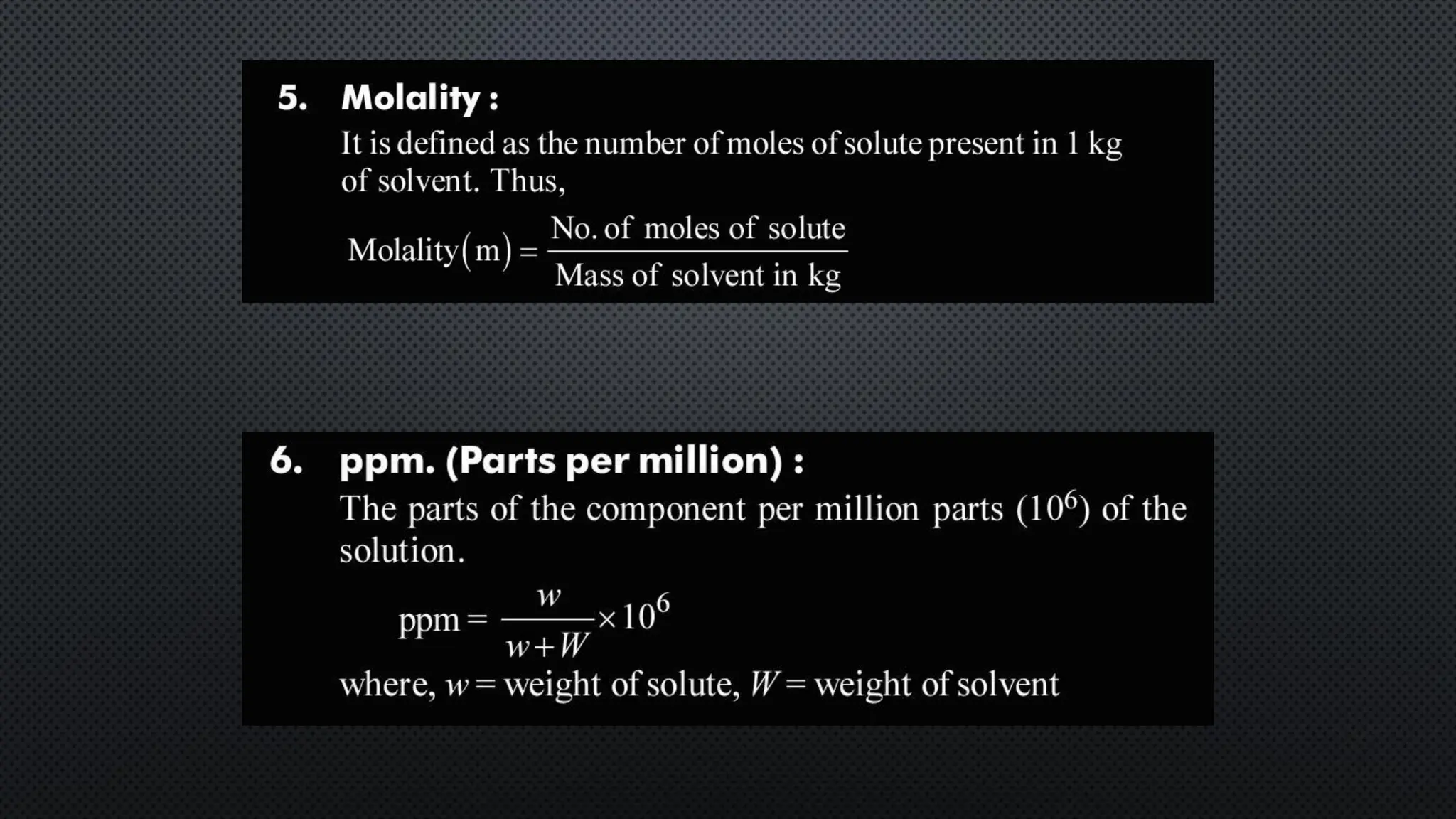
this pdf of notes was very important for those whon study in class 12 and this notes was made a 30 yr experience teaher for students and if any studen read this learn these notes properly . confirmly he or she scores best marks in exam thats all about this