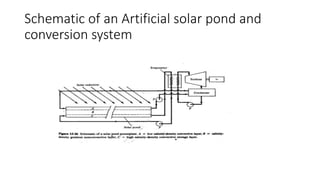
The document explains solar ponds, which collect and store solar energy through a salinity gradient that prevents convection currents, allowing heat to accumulate in the lower layer. It also describes photovoltaic energy conversion, which directly generates electricity from sunlight using solar cells made of semiconductor materials, primarily silicon. Furthermore, it touches on the solid-state principles and band theory that underpin electrical conduction in metals and semiconductors.









![• These valence electrons constitute primary mechanism of electric and heat
conduction in a metal. At the surface, however, there are no positive ions on
one side to give them equal attractive forces. Thus, while they are easily
moved by electric fields within the metal, they encounter an energy barrier at
the surface and require considerably more energy to get them out of the
metal.
• One therefore has an electron gas that is confined within the metal. It is not,
however, equivalent to an ordinary gas whose energy distribution given by the
Maxwell-Boltzmann law. Electrons, instead, exist in states restricted by the so-
called Pauli exclusion principle (which specifies that no two electrons in the
same atom can exist in the same state at the same time), and their energy
distribution is given by the Fermi-Dirac distribution law, given for temperatures
that are not too high (not greater than 3000 K) by [128]](https://image.slidesharecdn.com/solarponds-221009154311-6128570b/85/Solar-Ponds-pptx-11-320.jpg)



