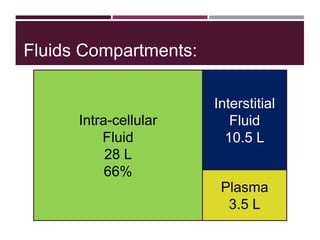
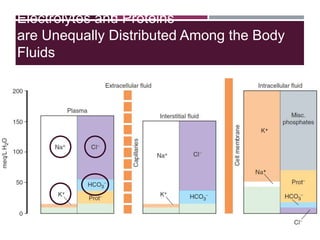
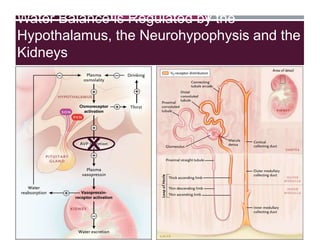
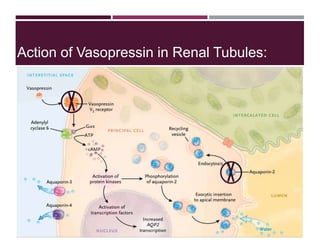
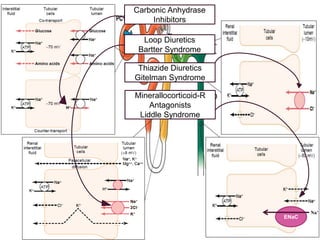
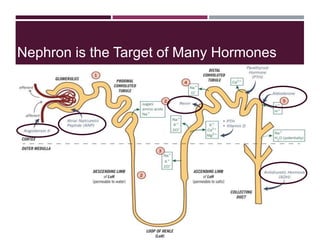
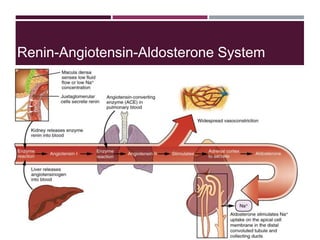
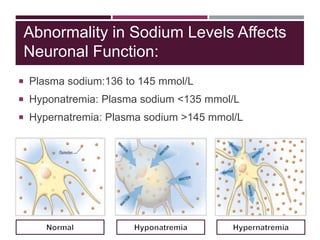
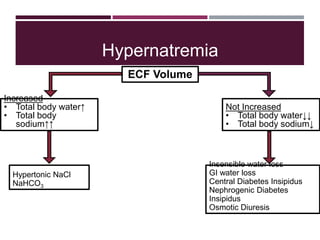
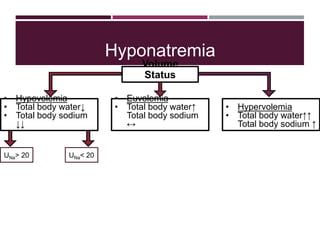
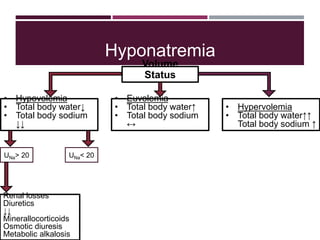
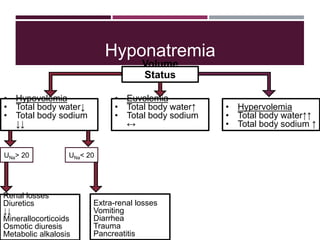
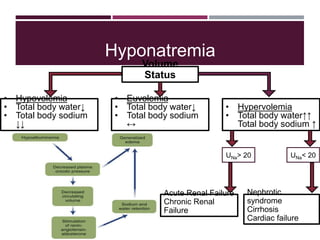
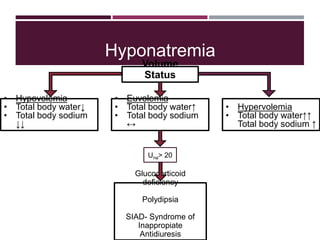
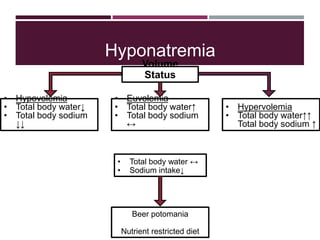
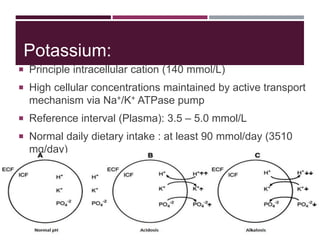
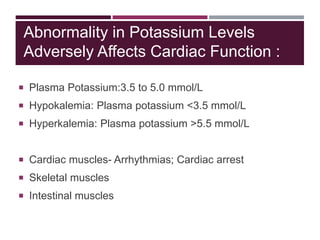
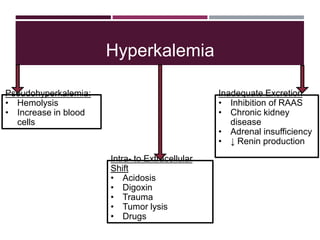
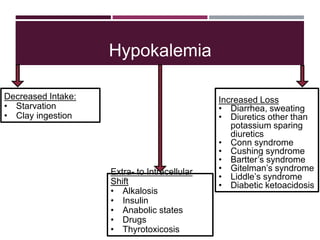
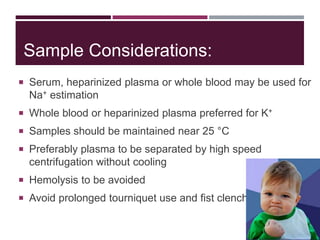
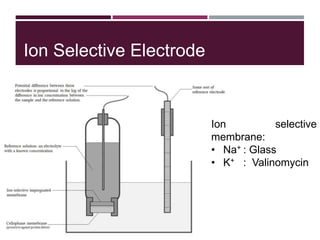
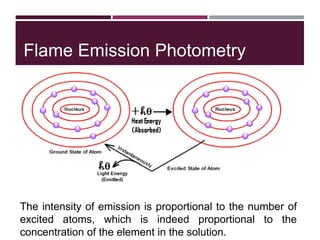
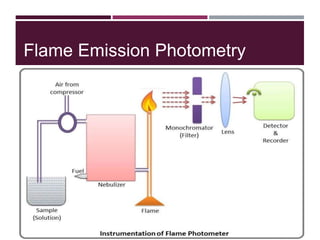
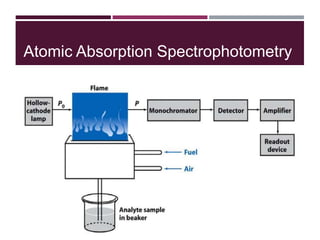
This document discusses sodium and potassium levels in the body. It begins by outlining the distribution of sodium, potassium, and water in the body and their roles in homeostasis. It then describes pathological conditions that can result from imbalances in sodium and potassium levels. Various techniques for estimating serum sodium and potassium are also outlined, including ion selective electrodes, atomic absorption spectroscopy, and flame emission photometry.