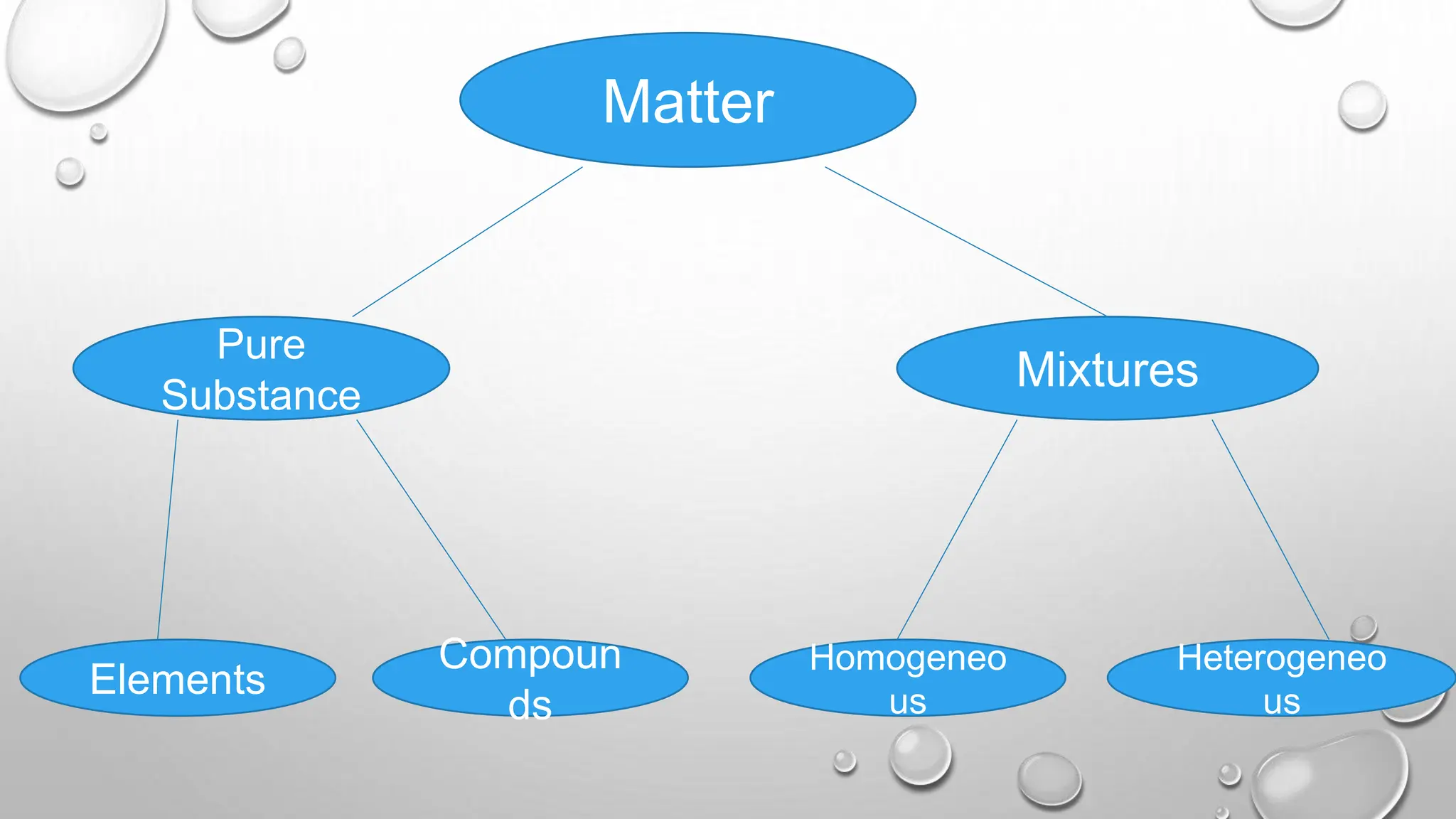
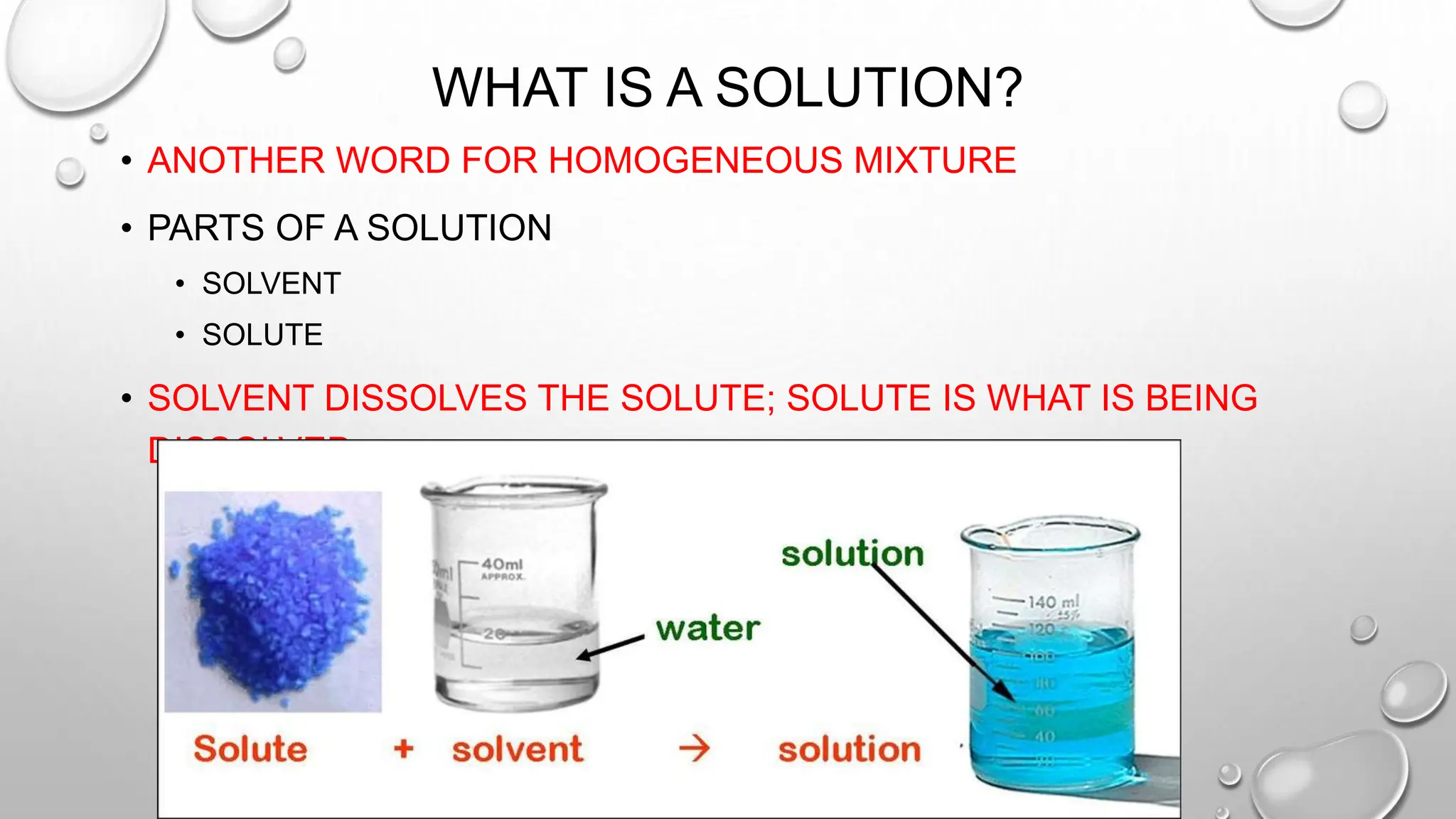
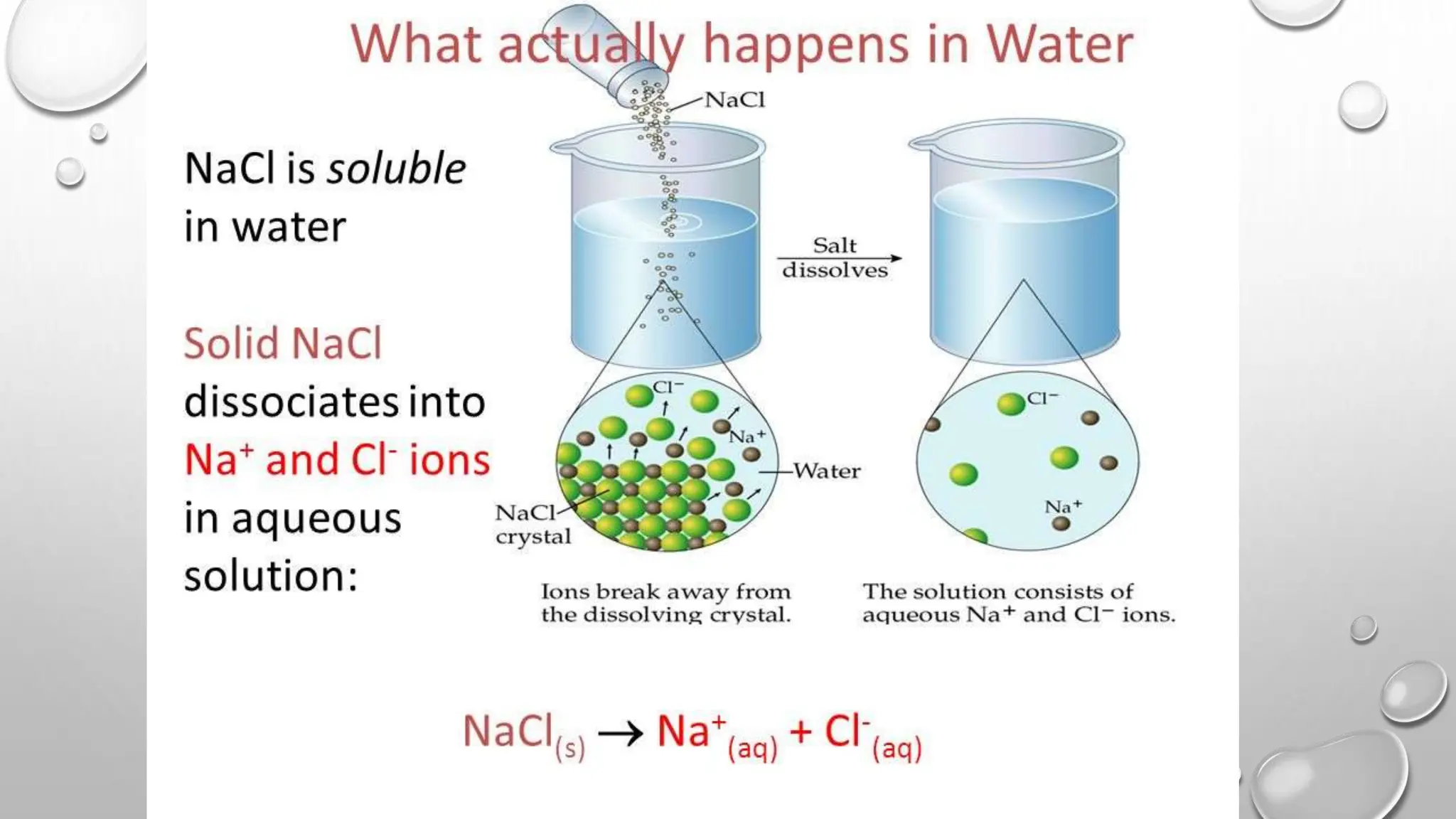
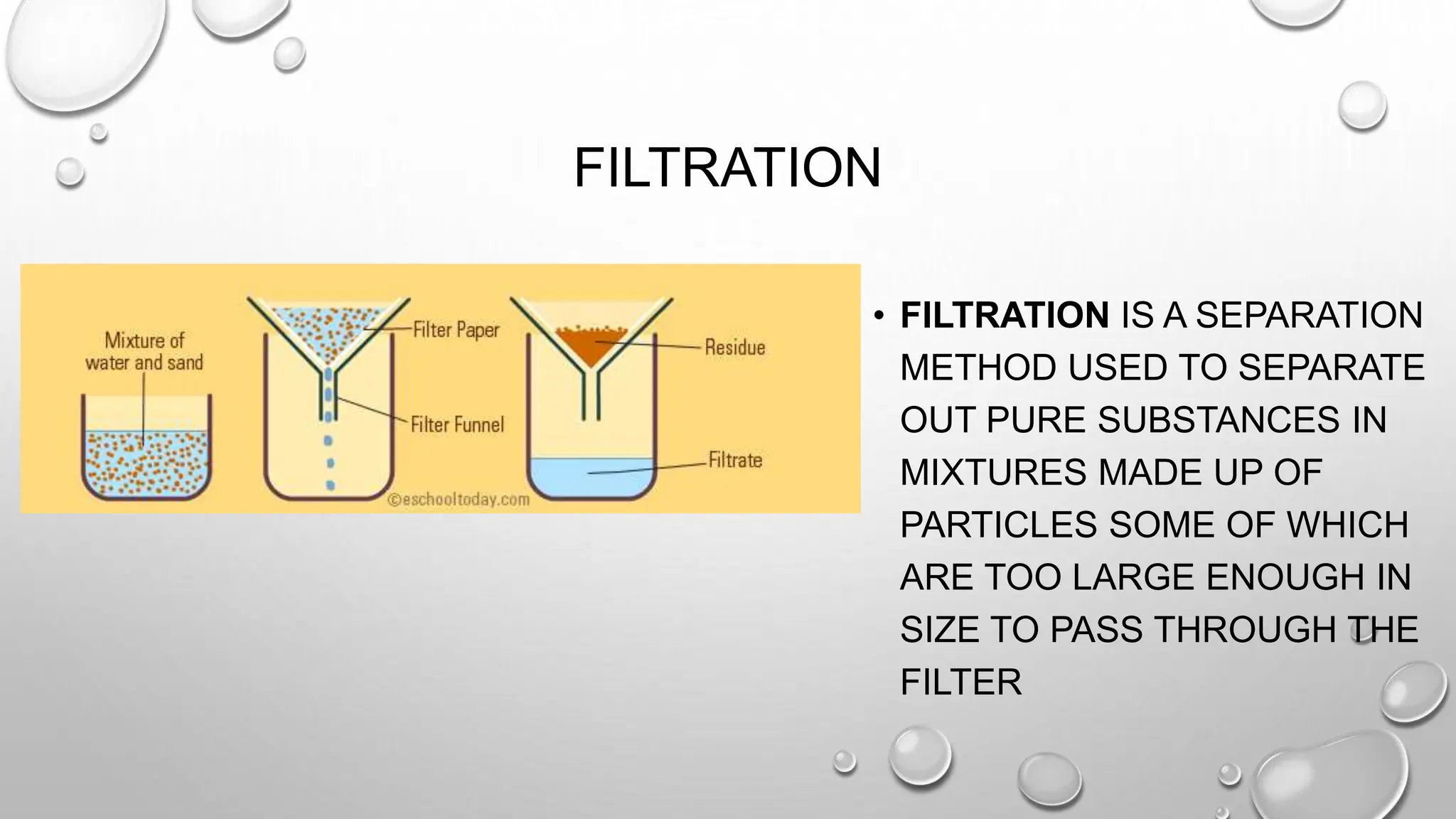
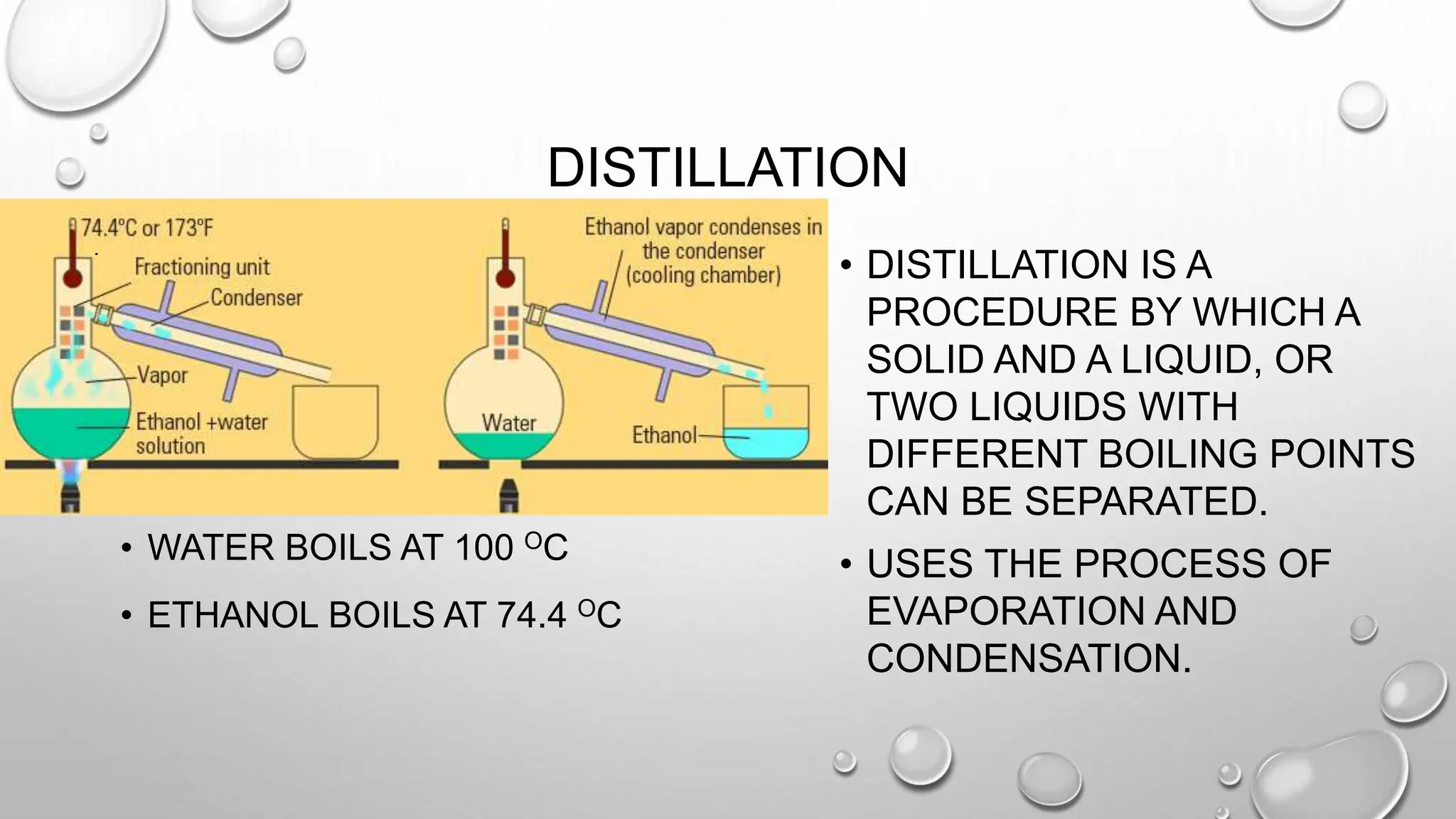
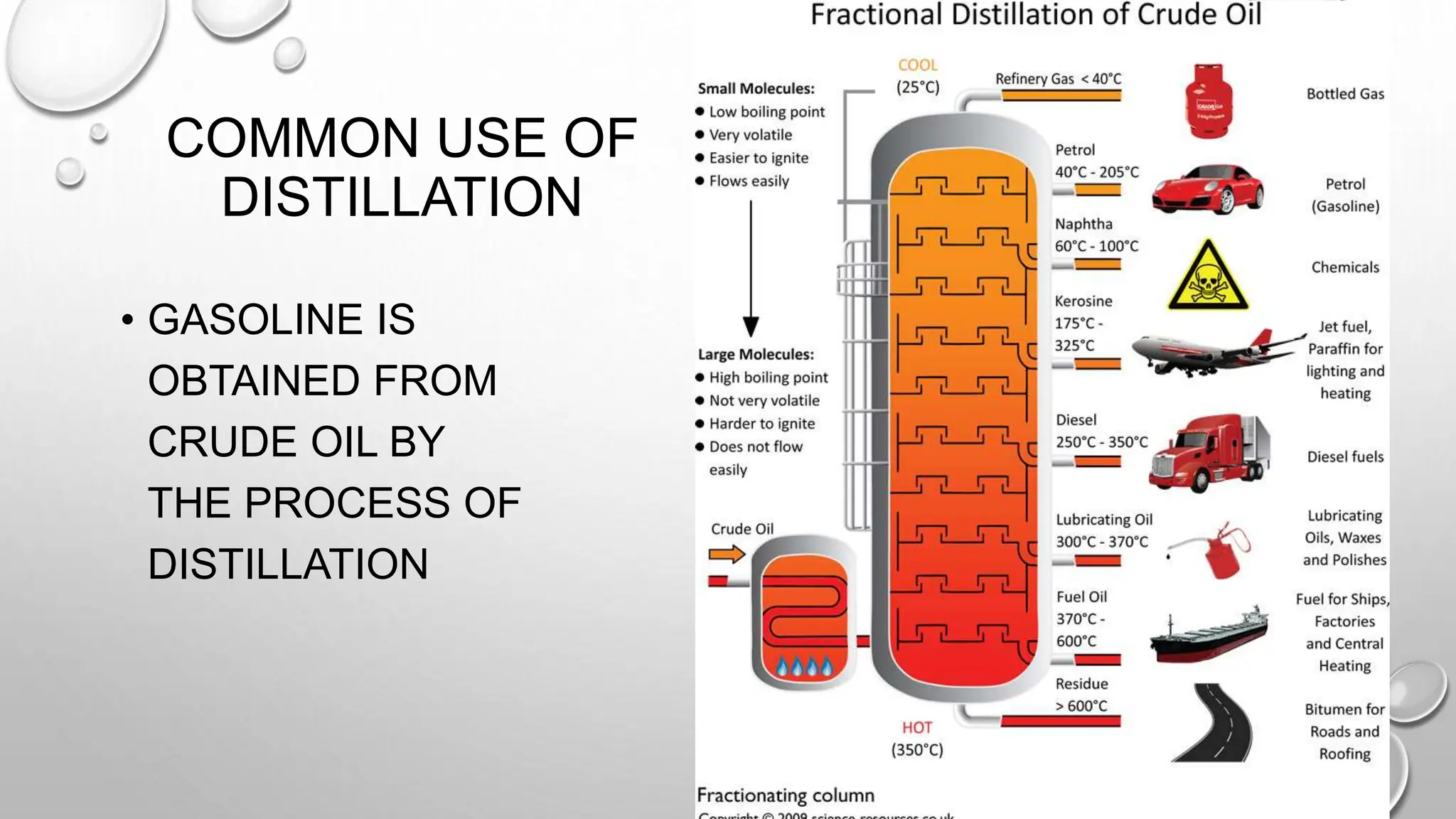
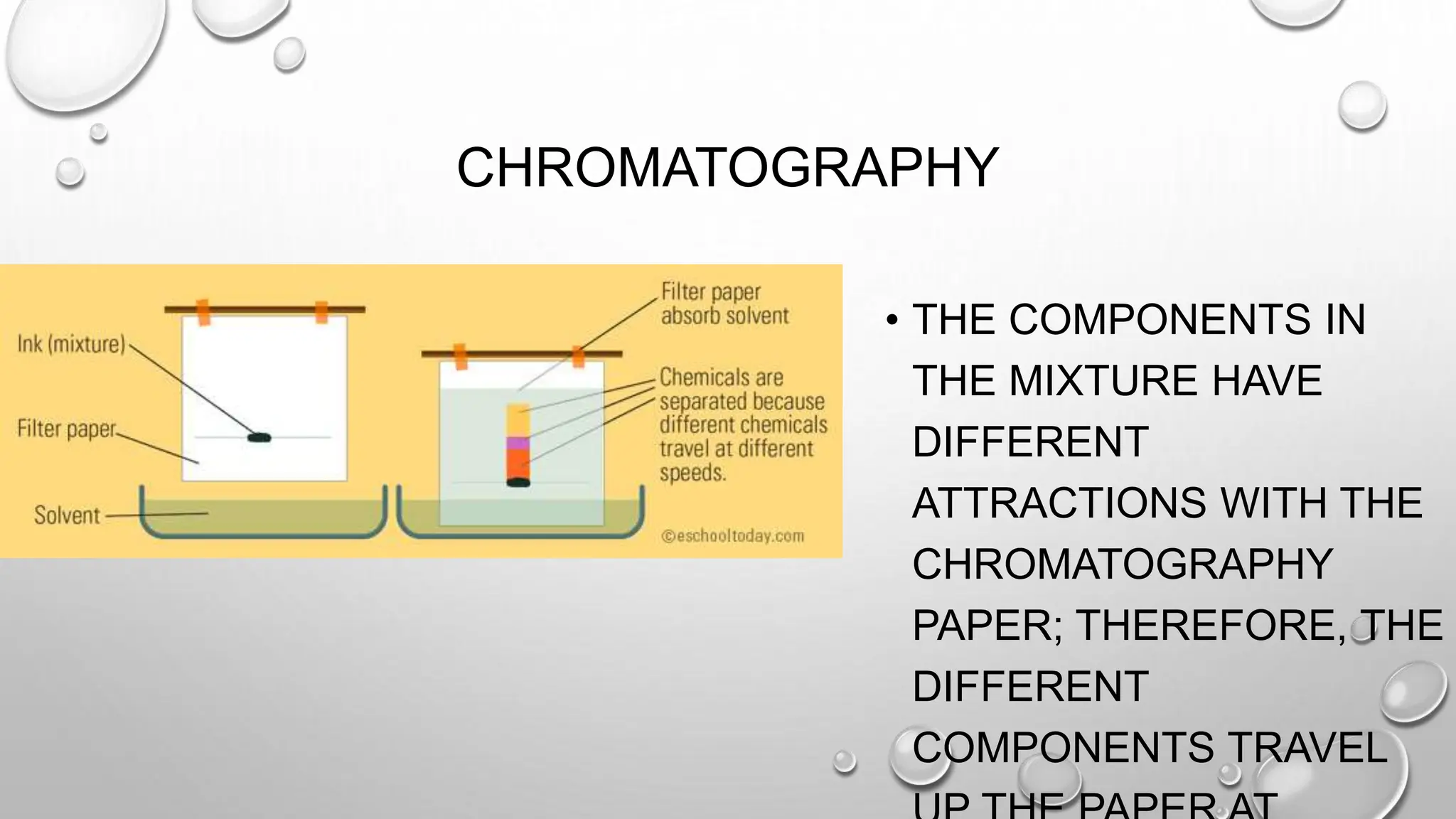
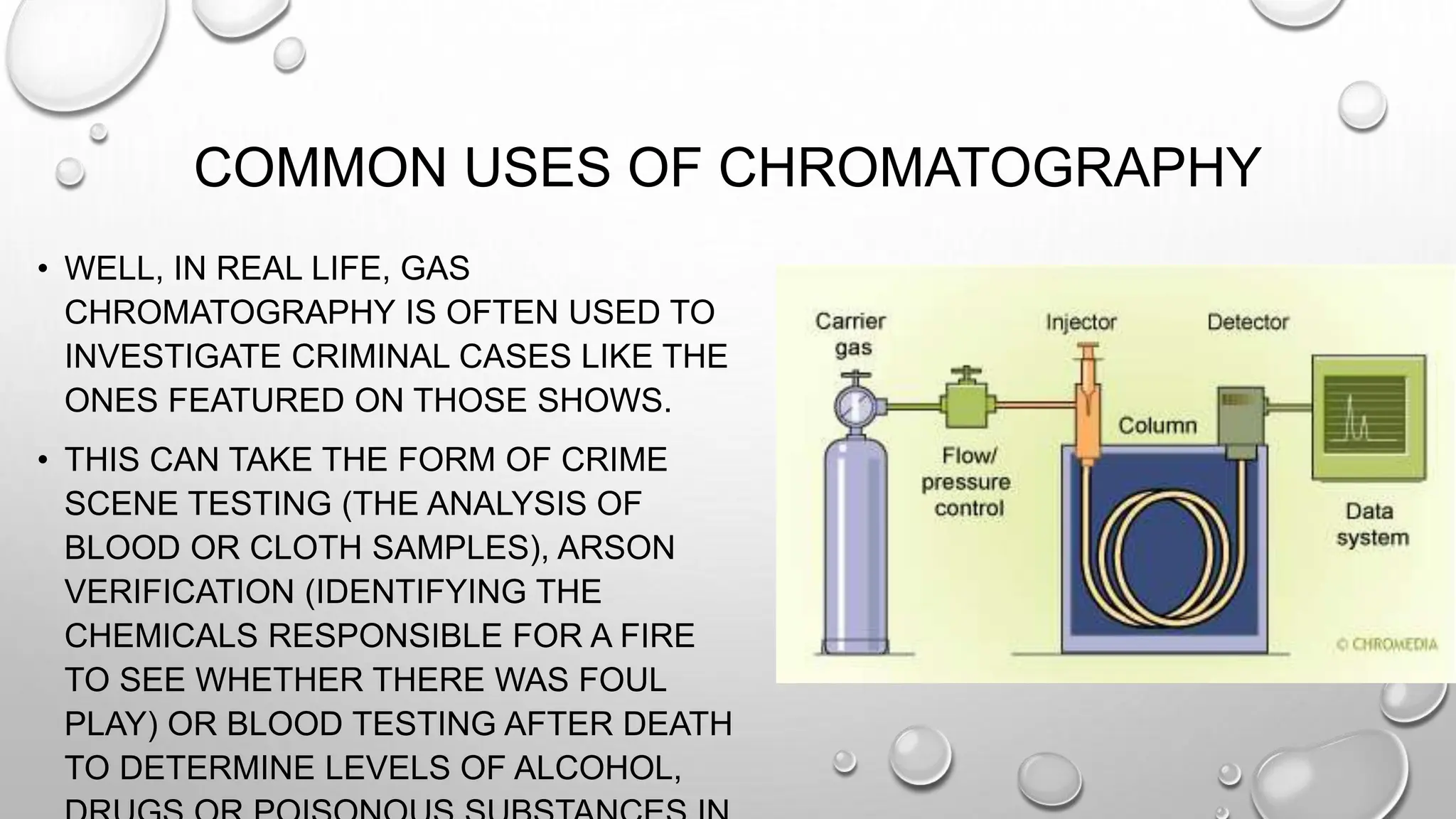
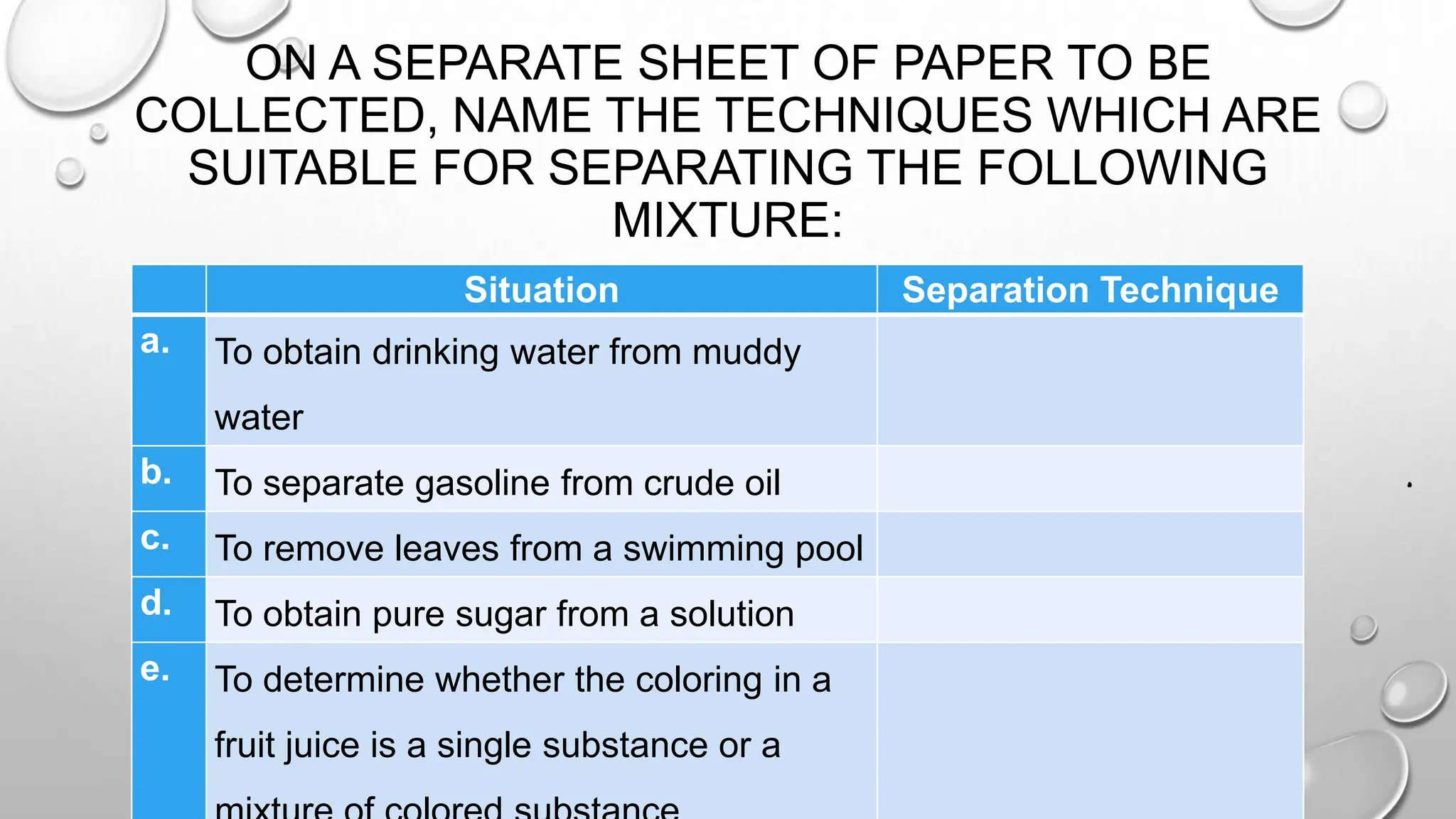
The document outlines the differences between compounds and mixtures, defining key terms and providing examples. It explains separation methods including filtration, distillation, and chromatography, highlighting their processes and common uses. The document concludes with an exercise for students to identify appropriate separation techniques for specific scenarios.