The document discusses semen analysis, including:
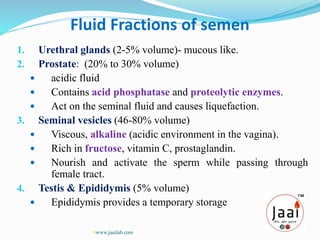
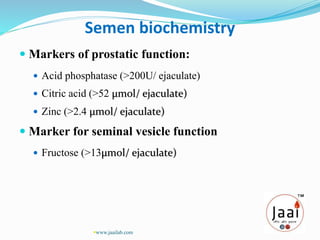
1. It describes the different fractions that make up semen and their functions, such as nourishing sperm.
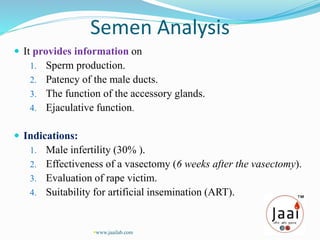
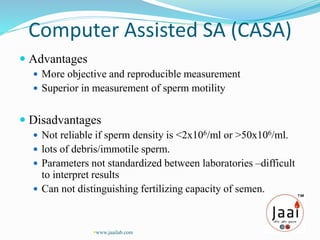
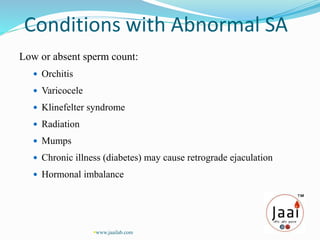
2. Semen analysis provides information on sperm production, male duct patency, accessory gland function, and ejaculation. It is used to evaluate male infertility, vasectomy effectiveness, and artificial insemination suitability.
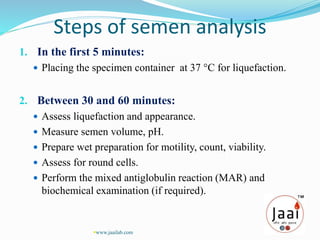
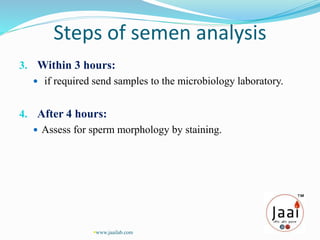
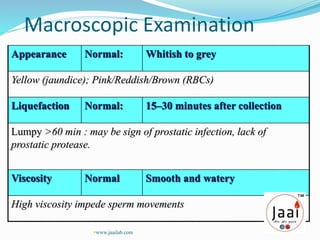
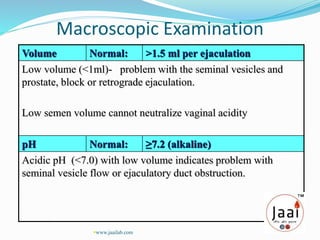
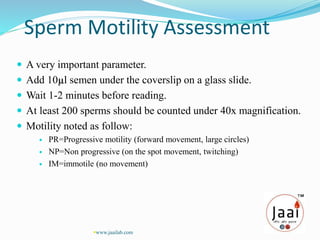
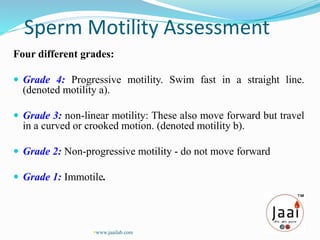
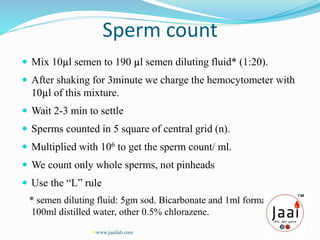
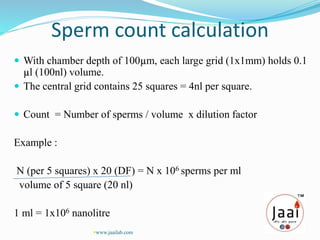
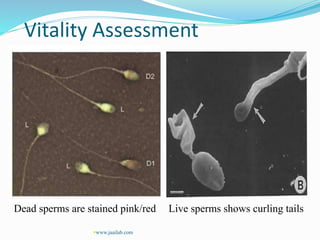
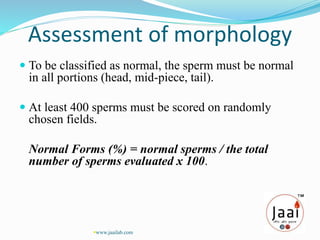
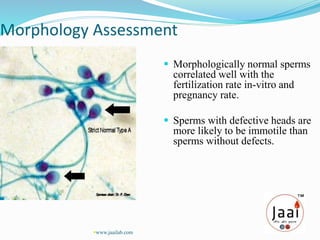
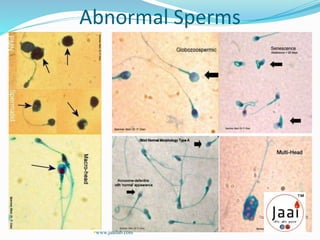
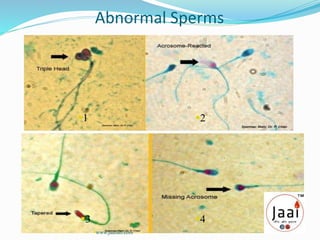
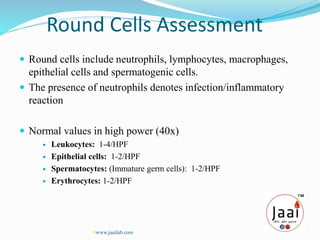
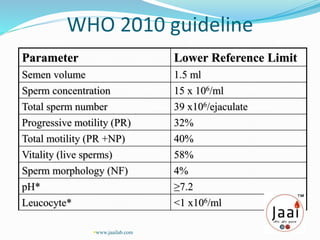
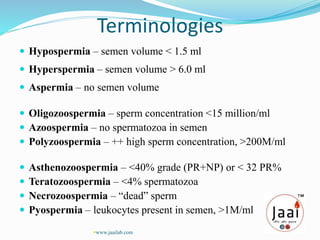
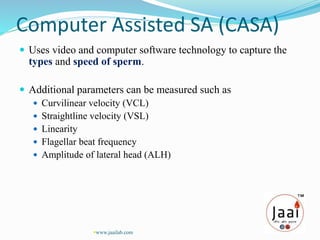
3. Parameters examined in semen analysis include volume, pH, sperm count, motility, morphology, and presence of round cells. Tests are performed within a few hours of collection to assess these parameters.
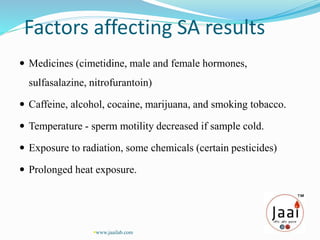
4. Multiple factors can affect semen analysis results, so repeated testing is often needed for an accurate assessment