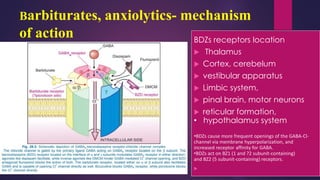
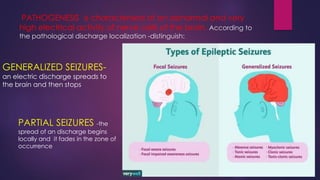
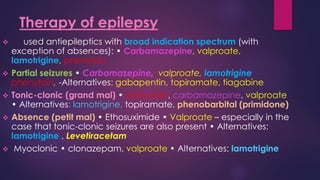
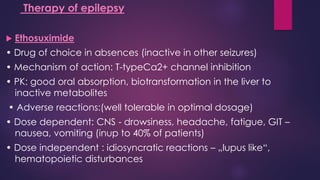
The document outlines various classes of psychotropic drugs utilized for treating mental disorders, including neuroleptics, anxiolytics, and sedative-hypnotics. It details the mechanisms of action, therapeutic uses, and pharmacokinetics of sedatives, particularly barbiturates and benzodiazepines, as well as their side effects and classifications. Additionally, it discusses the therapeutic applications of these drugs in managing conditions like anxiety, insomnia, and seizure disorders.