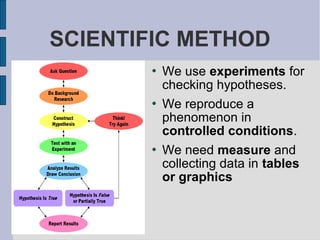
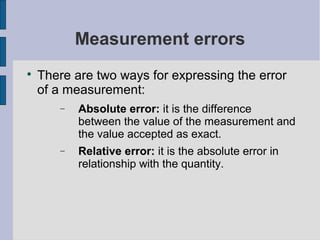
The document discusses physics and chemistry, comparing what they have in common (studying matter) and what makes them different (physics studies phenomena that don't change matter composition, while chemistry studies phenomena that do change composition). It then provides an overview of the scientific method, including making observations and asking questions, developing hypotheses to test, conducting controlled experiments, analyzing results, and drawing conclusions. Finally, it covers scientific concepts like units, measurements, errors, and notation.