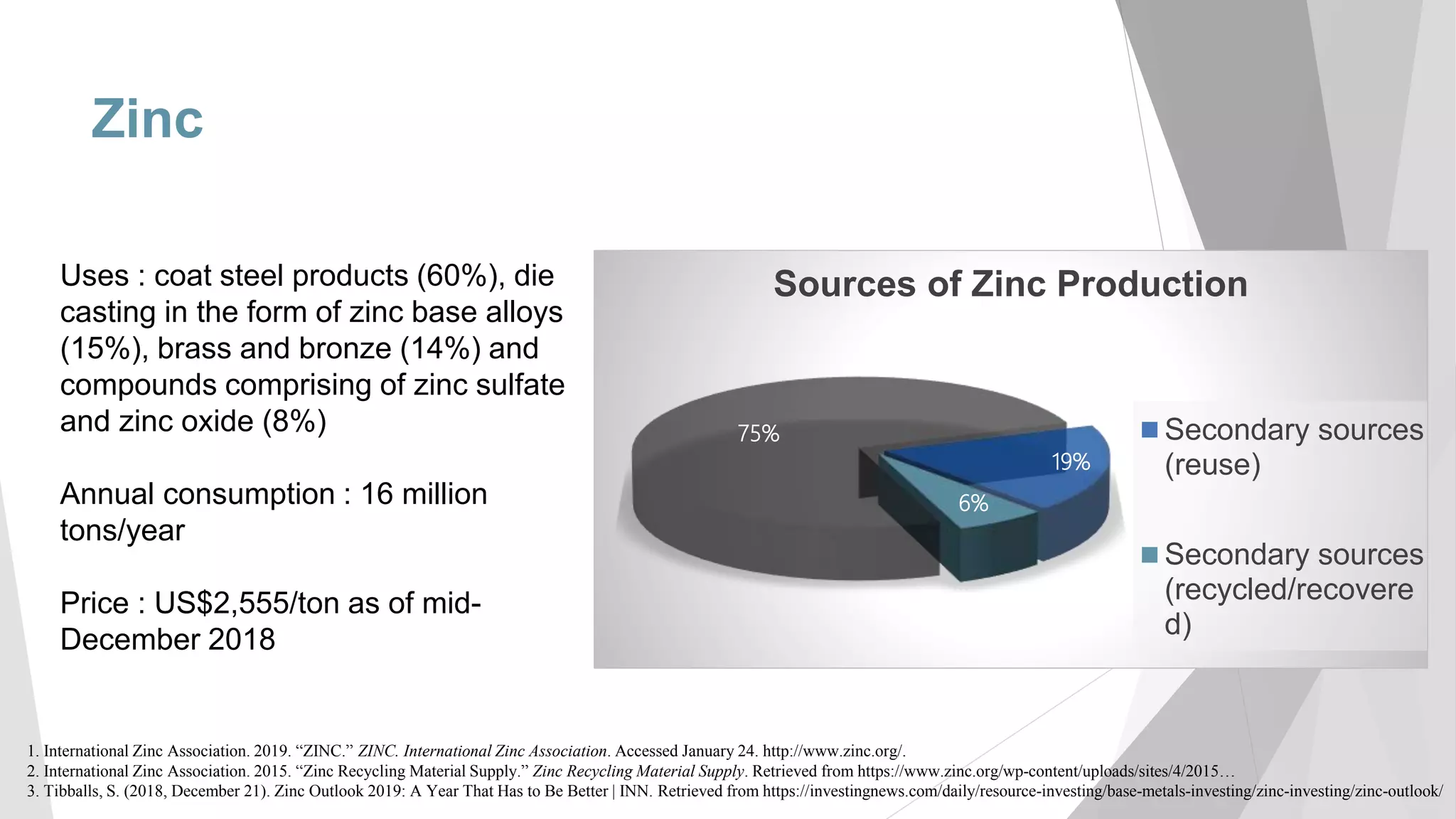
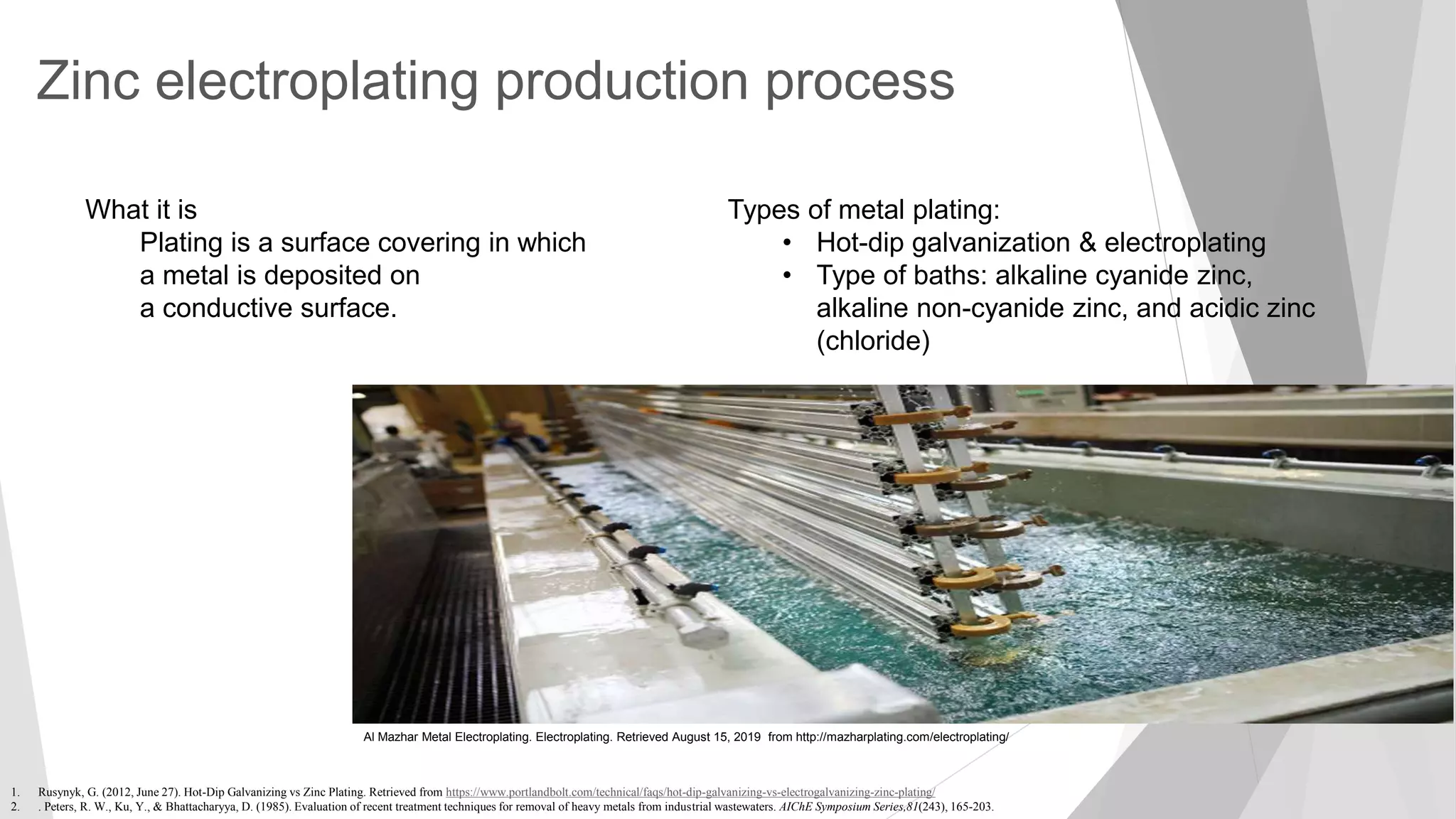
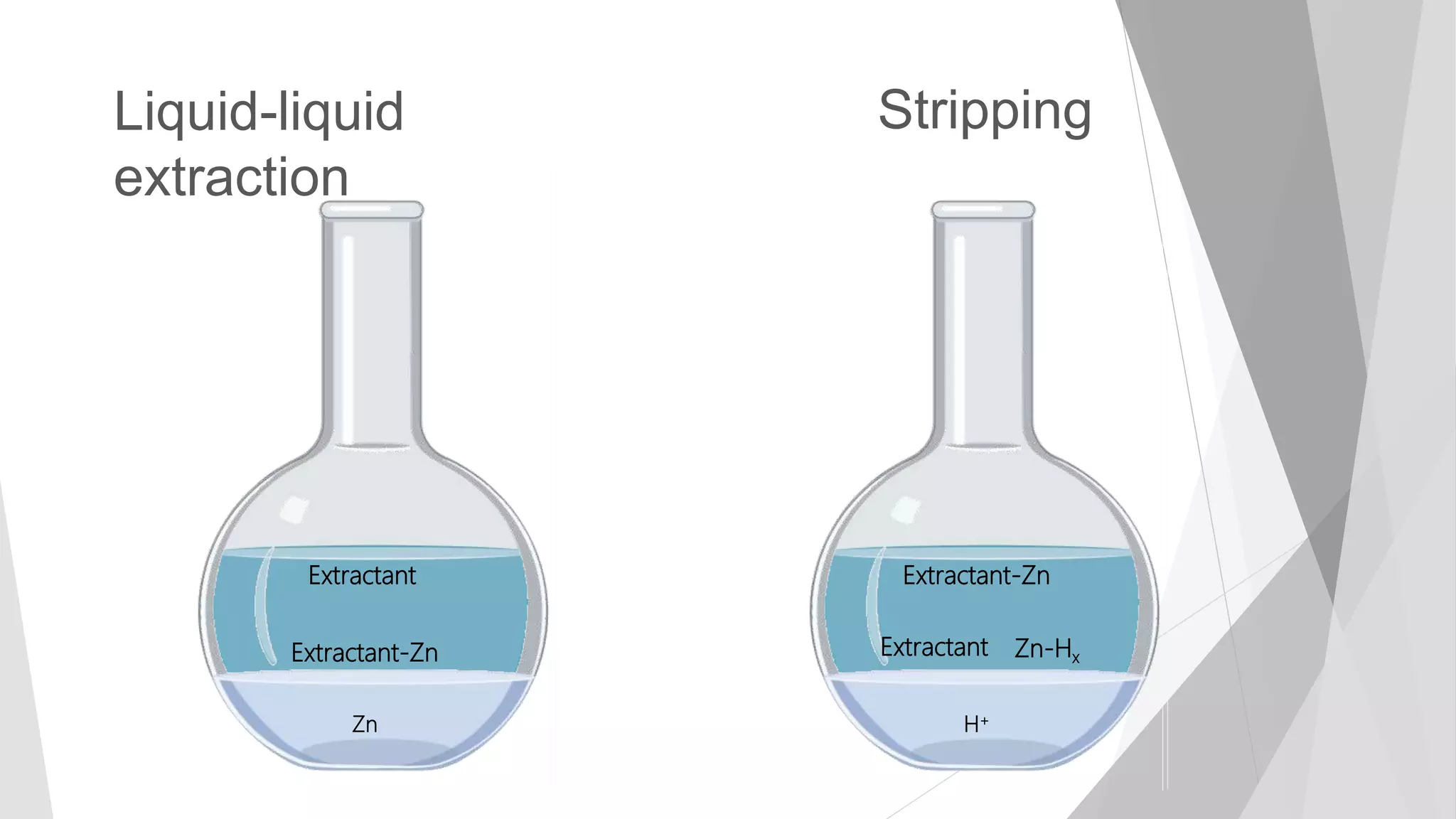
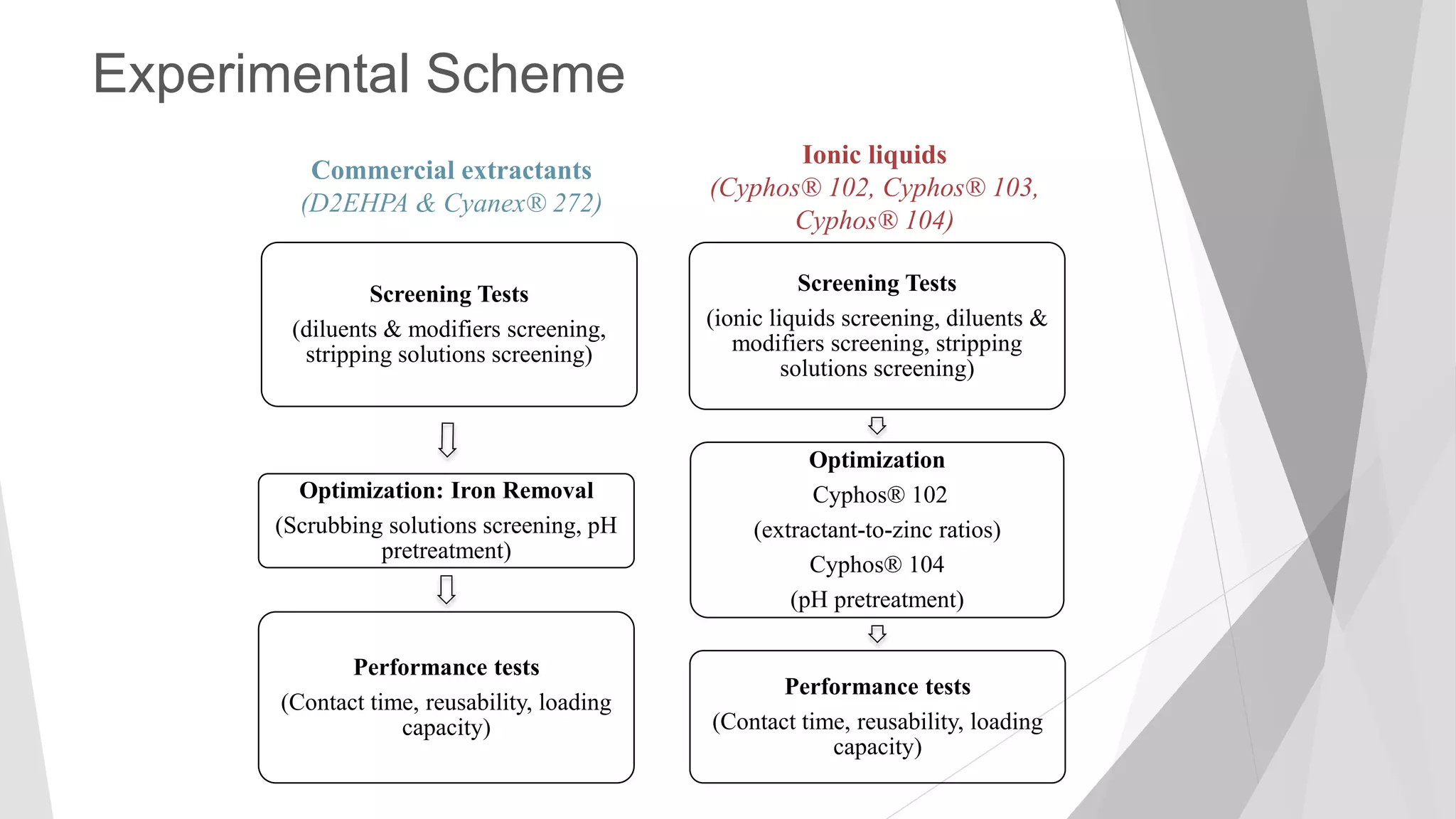
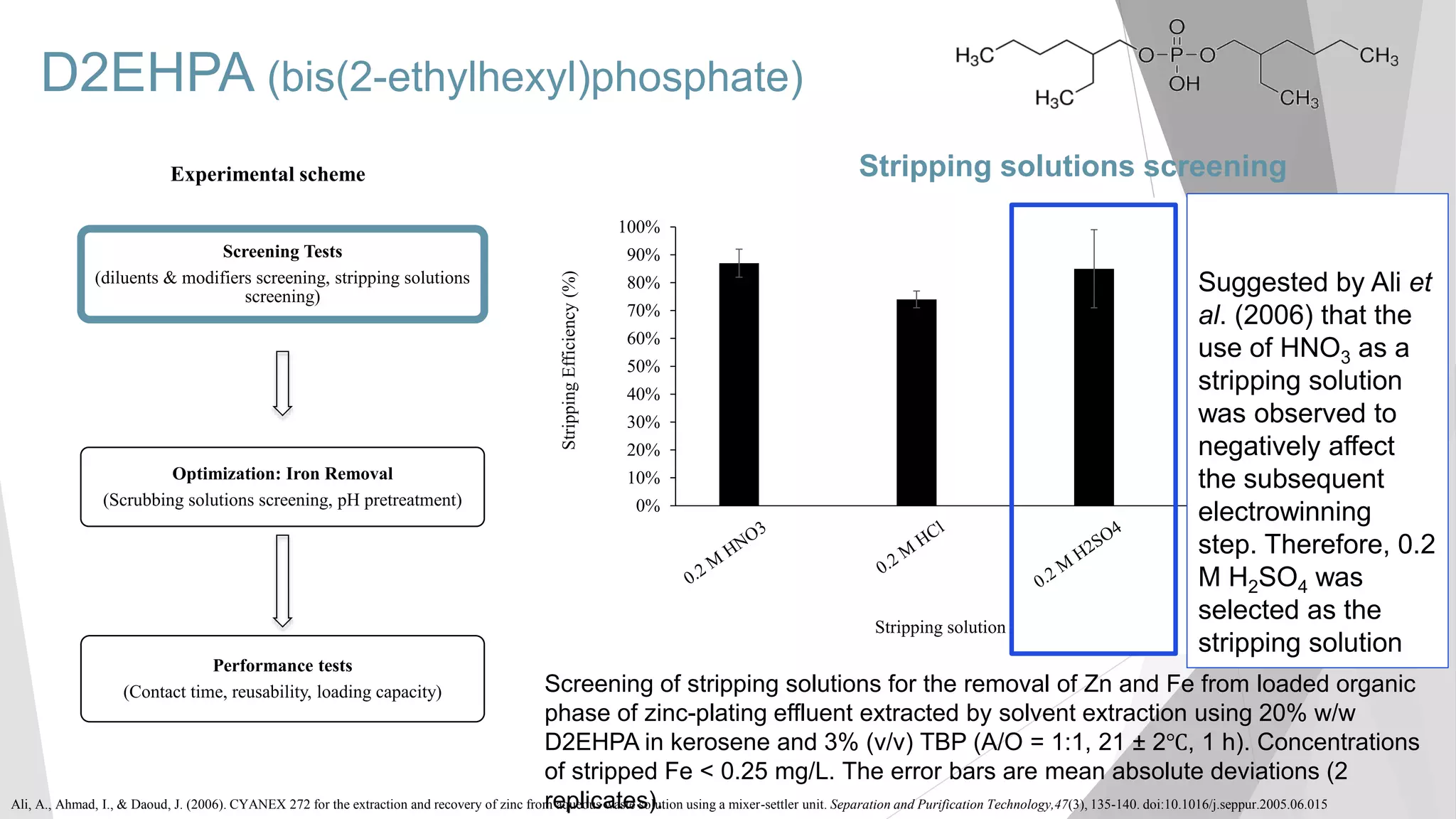
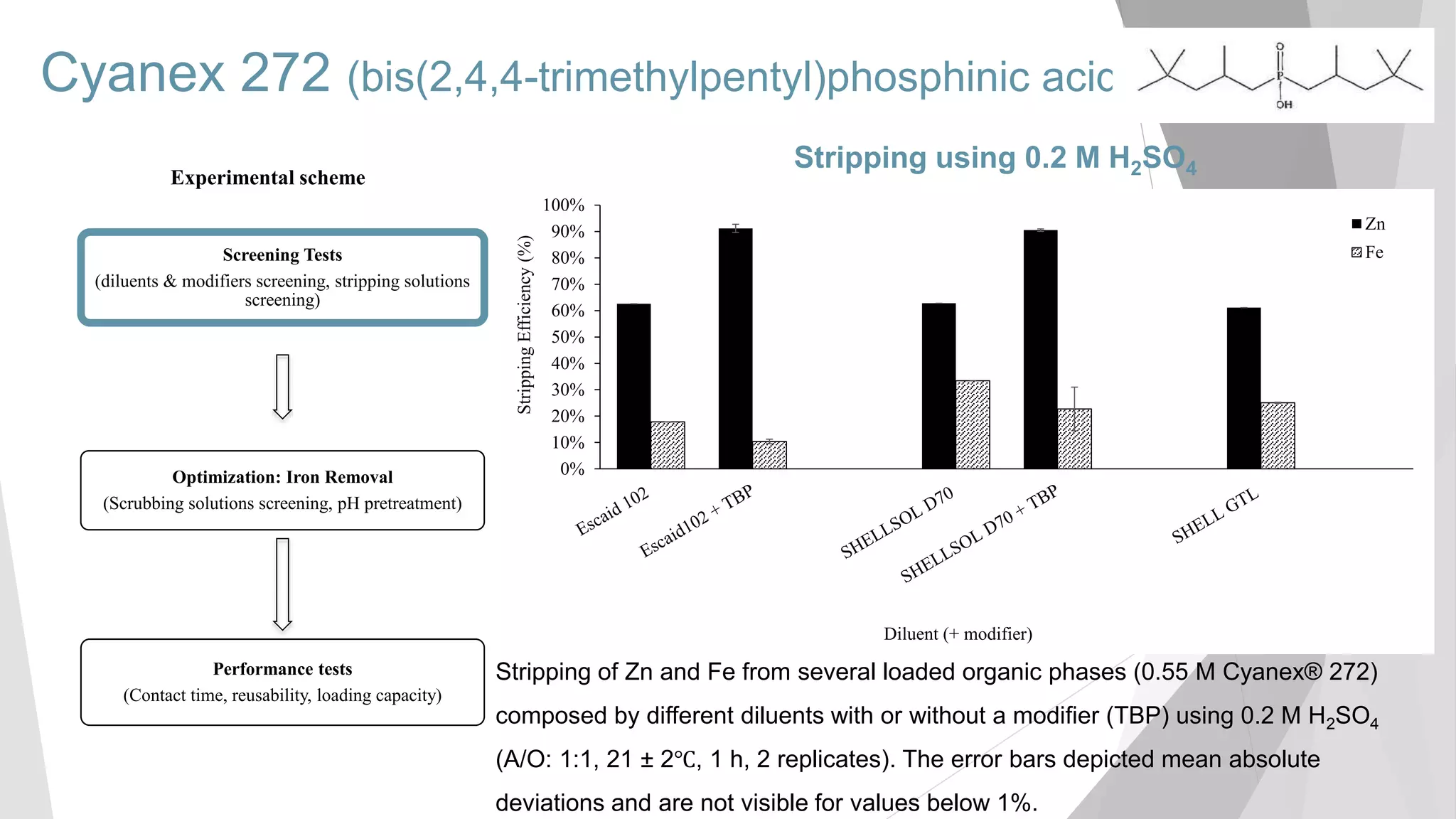
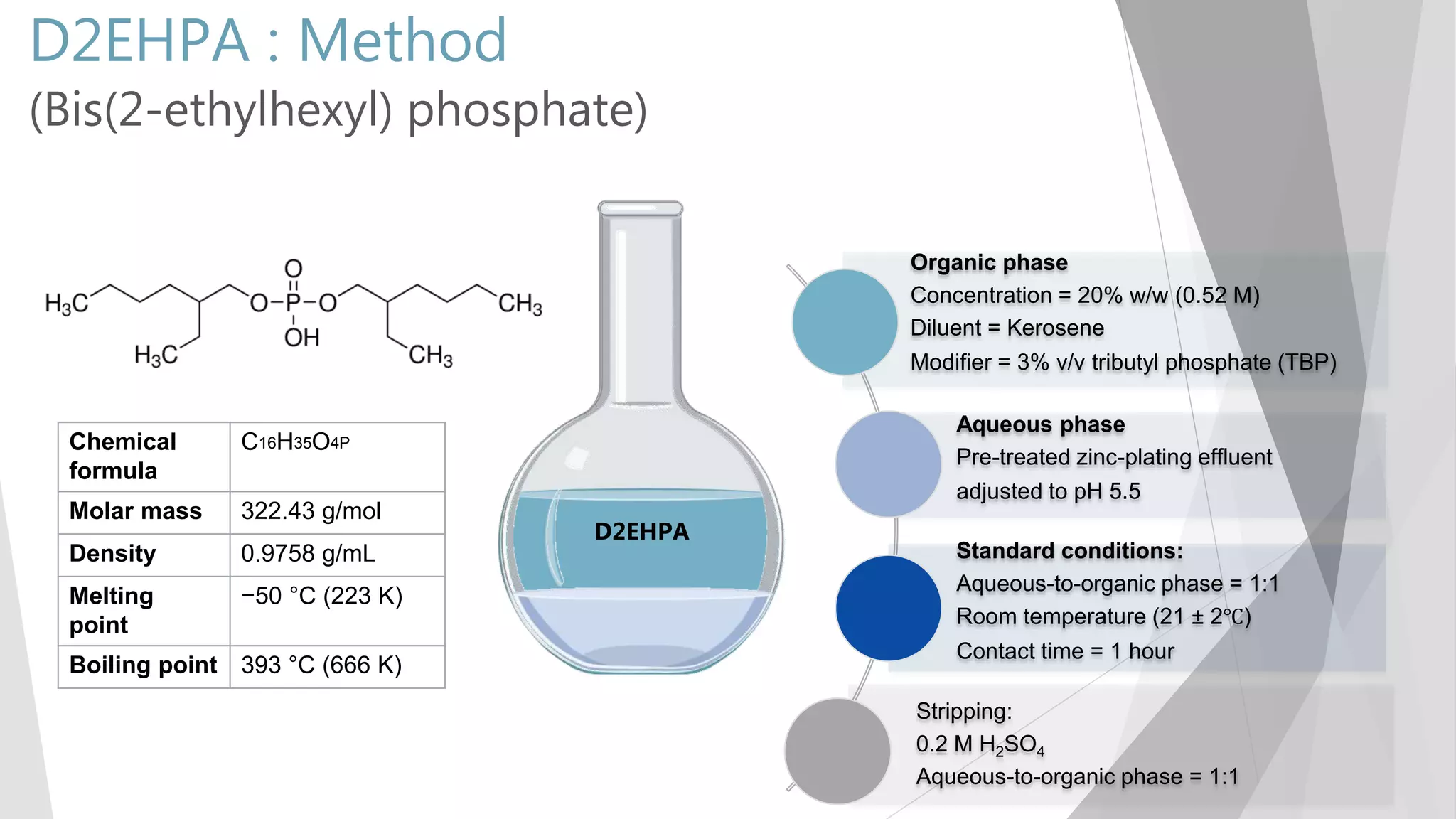
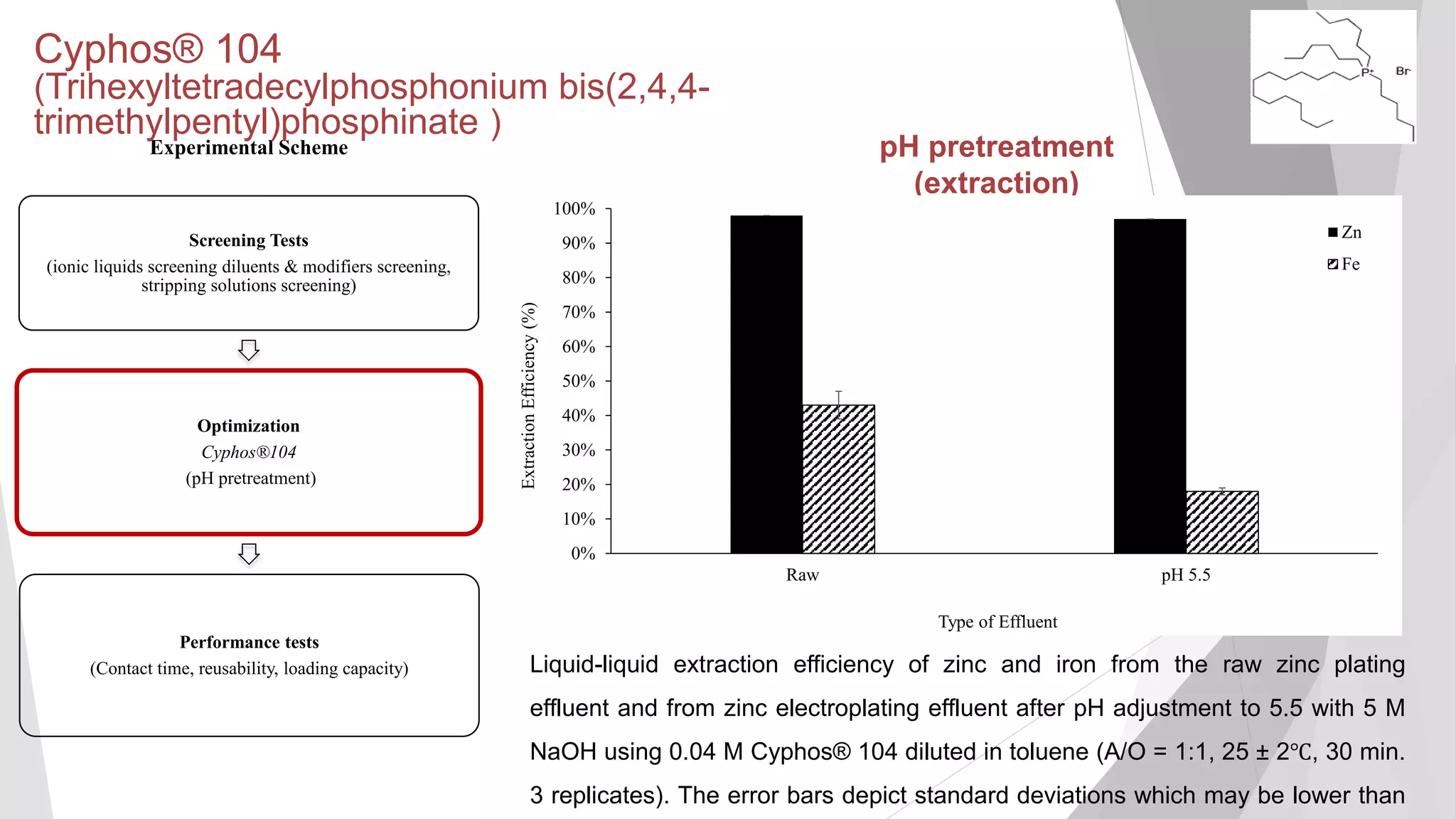
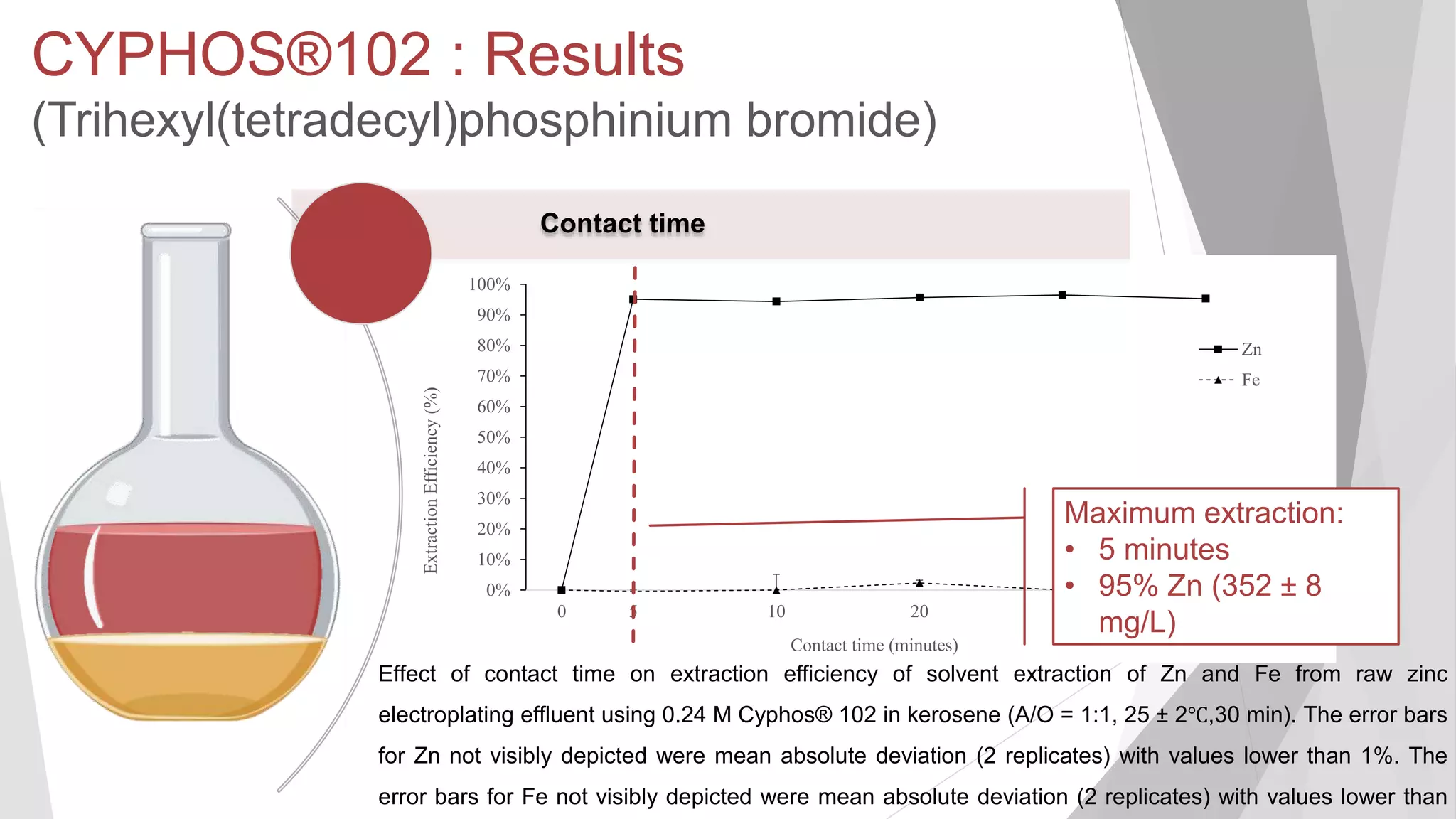
The document discusses zinc production and its various uses, highlighting that 60% goes toward coating steel products and detailing secondary recycling sources. It covers different electroplating methods, wastewater characterization, and the effectiveness of using solvent extraction techniques like d2ehpa for recovering zinc from plating effluents. Additionally, it mentions challenges in solvent extraction and the importance of factors such as pH and contact time for optimizing the recovery process.














inorganicphase
[Zn] (mg/L) in aqueous phase
Loading Capacity
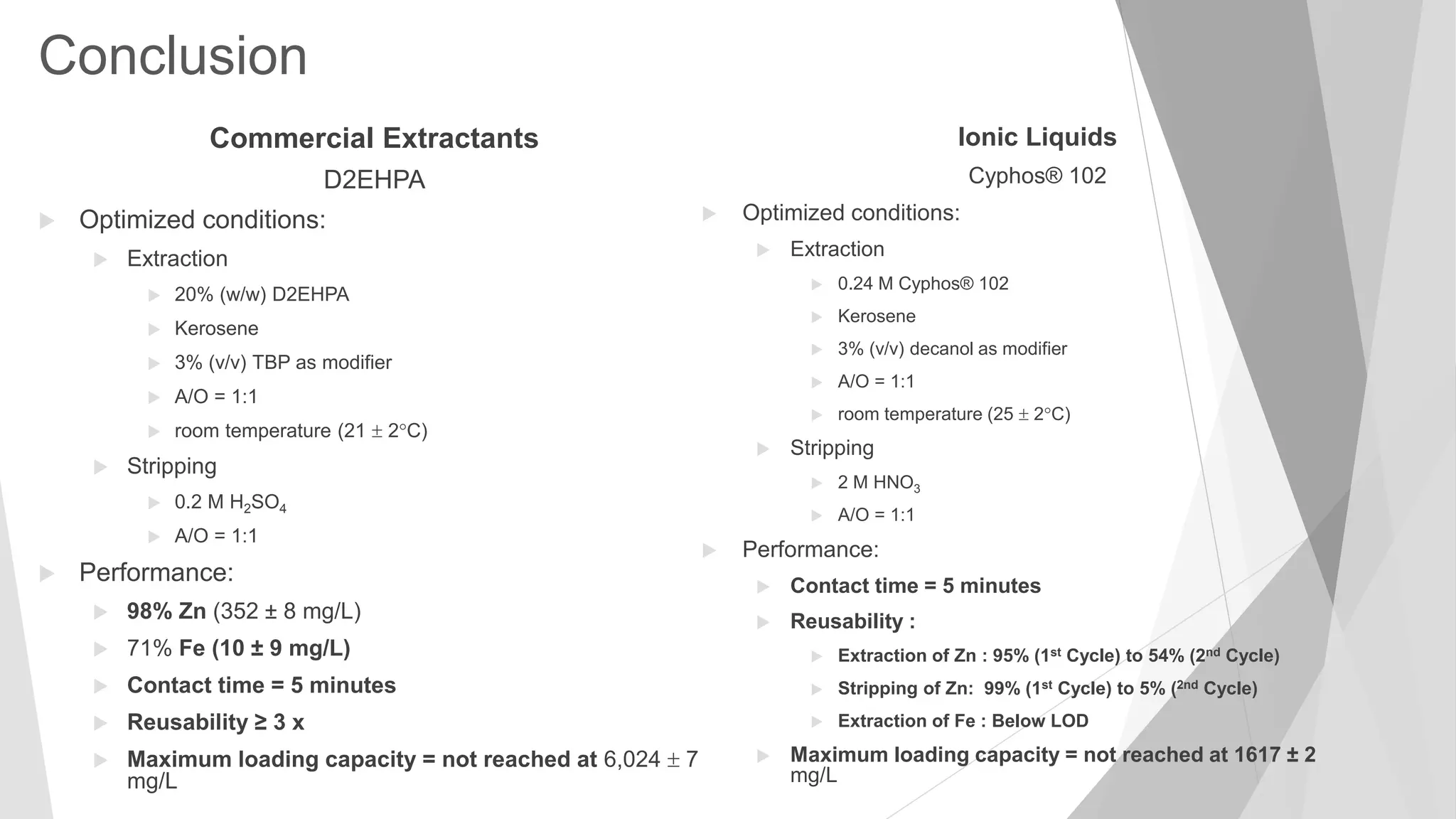
D2EHPA
D2EHPA : Results
(Bis(2-ethylhexyl) phosphate)
Maximum loading capacity not
reached even after A/O ratio of
35:1 (loading capacity of 6,024
7 mg/L)
Equilibrium isotherm (loading capacity) of Zn extraction of pretreated zinc-plating effluent (pH 5.5)
at 21±2°C using 20% (w/w) D2EHPA in kerosene with 3% (v/v) TBP as modifier (21 ± 2℃, 5 min.,
2 replicates). The error bars depict mean absolute deviations (smaller than the symbols in most](https://image.slidesharecdn.com/sathityatiwatchirthesisdefensefinal-200108033836/75/Sathityatiwat-ChIR-thesis-defense-22-2048.jpg)





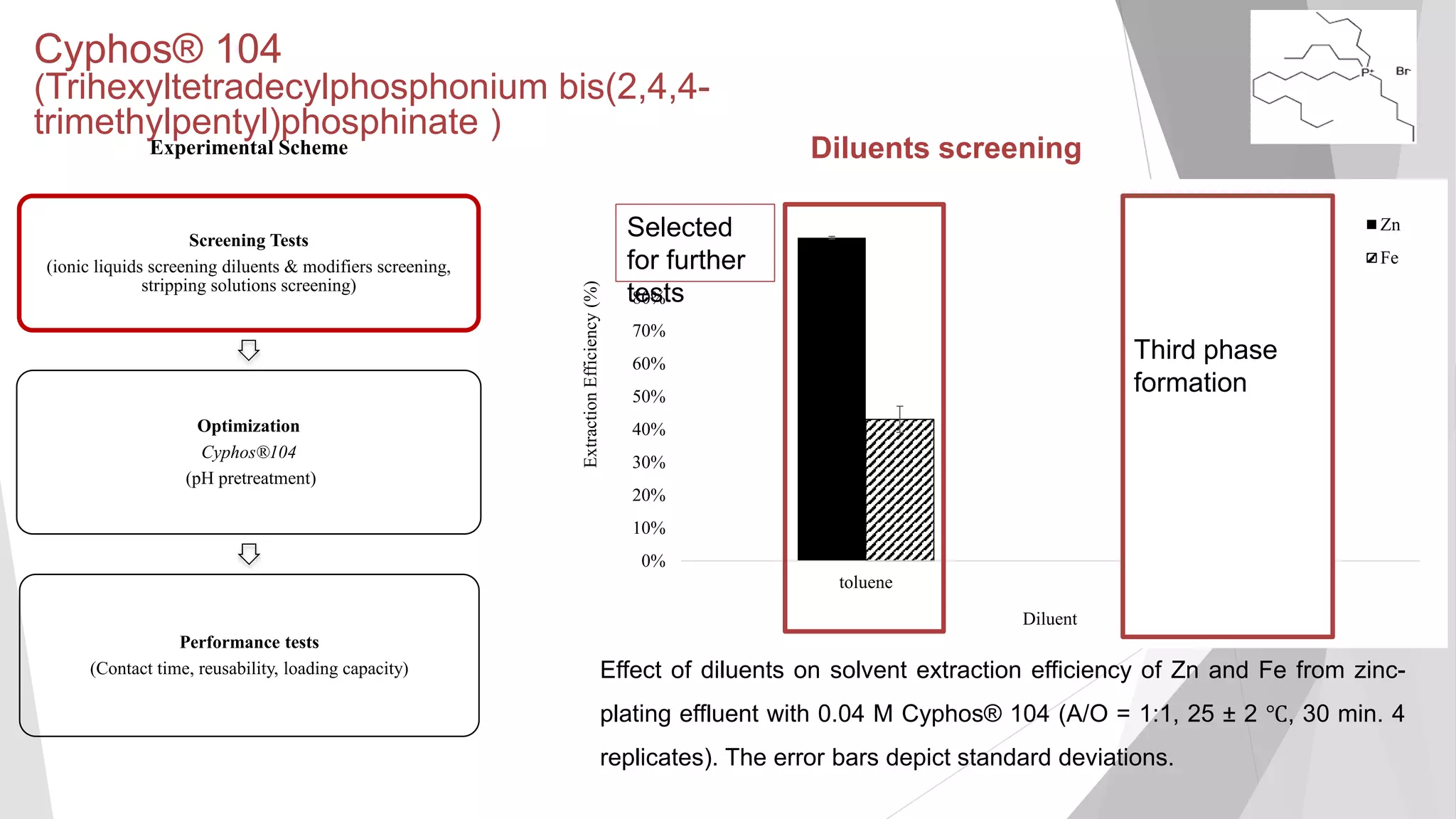
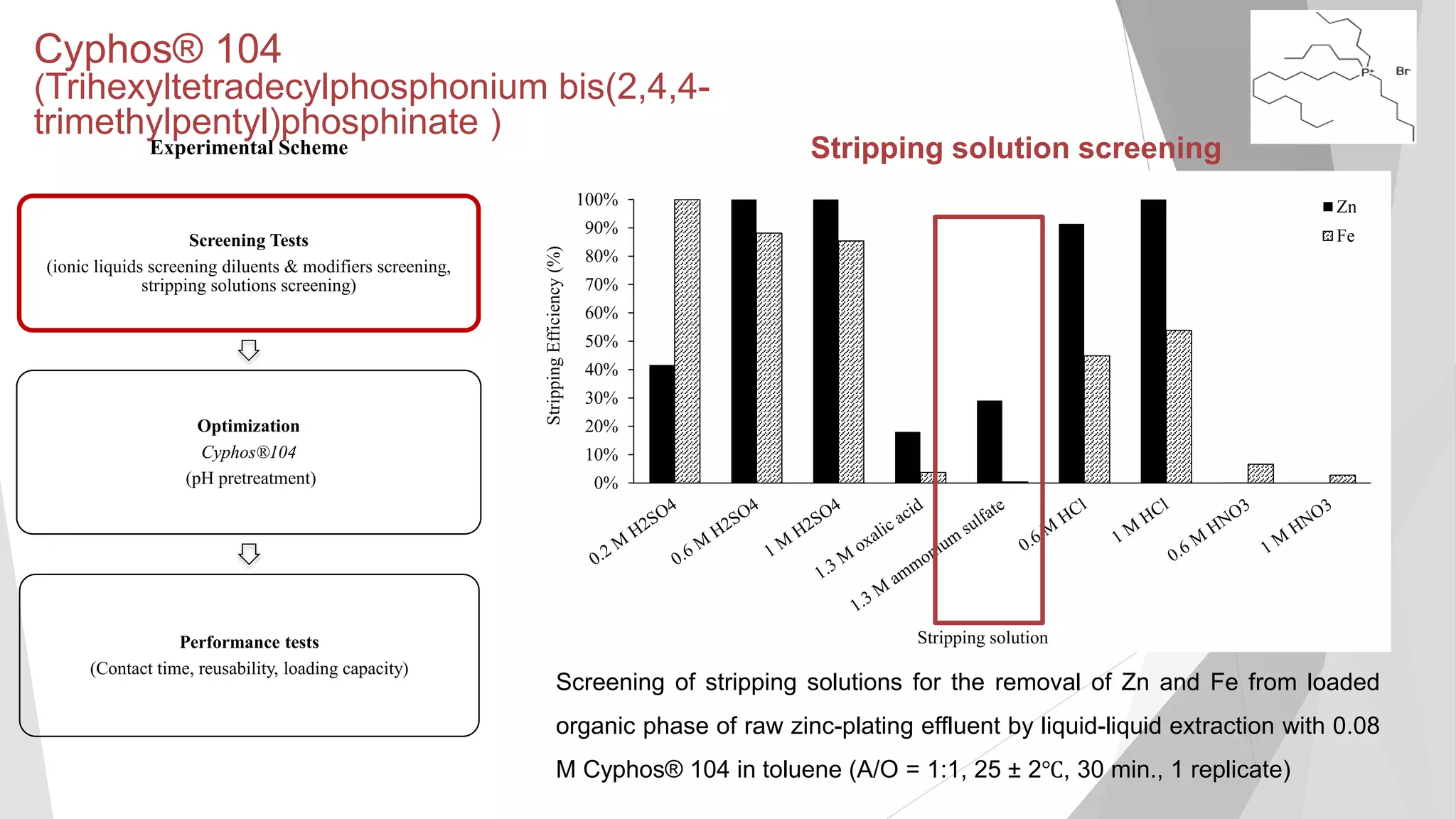

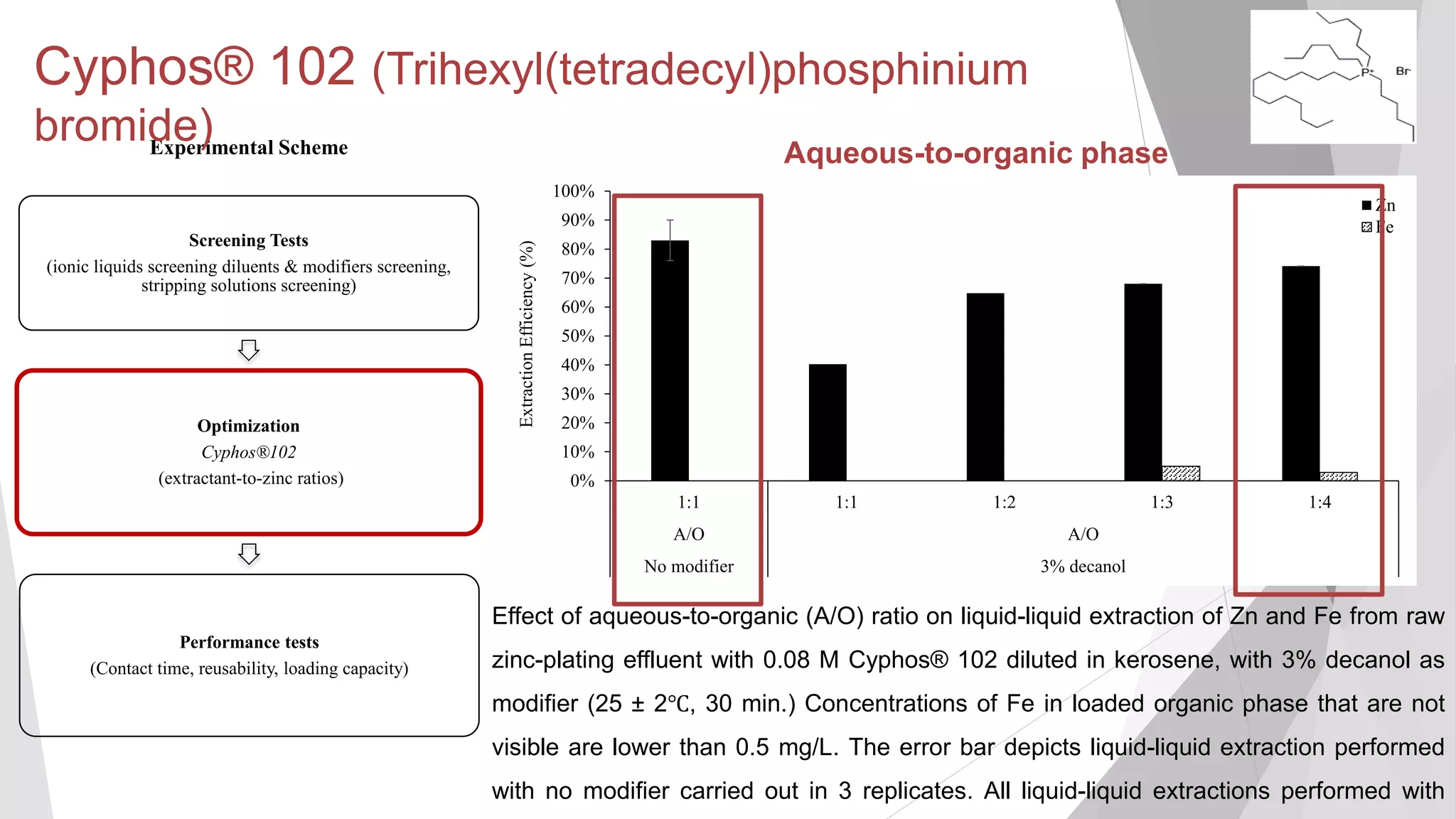





inorganicphase
[Zn] (mg/L) in aqueous phase
Equilibrium isotherm of Zn extraction of raw zinc-plating effluent at 25±2°C using 0.24
M Cyphos® 102 in kerosene with 3% (v/v) decanol as modifier (25 ± 2℃, 130 min.).
The error bars not visibly depicted were mean absolute deviation (2 replicates) of less
Maximum loading capacity not
reached even after A/O ratio of
10:1 (loading capacity of 1,617
2 mg/L)](https://image.slidesharecdn.com/sathityatiwatchirthesisdefensefinal-200108033836/75/Sathityatiwat-ChIR-thesis-defense-40-2048.jpg)