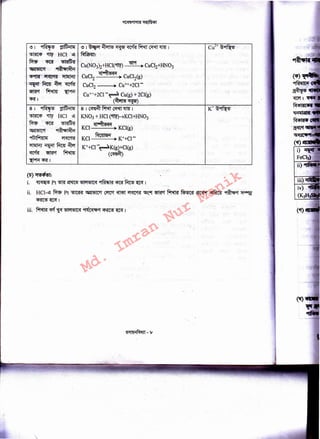
This document describes several chemical reactions involving the addition of hydrochloric acid (HCl) to various salts. It provides the chemical equations for the reactions of HCl with sodium chloride (NaCl), calcium chloride (CaCl2), potassium nitrate (KNO3), and copper(II) nitrate [Cu(NO3)2]. The reactions produce the corresponding hydrogen chloride salts and nitric acid as products. The document also discusses the use of these acid-base reactions to observe the movement of cations (Na+, Ca2+, K+, Cu2+) between the ground state and aqueous state using indicator solutions.

![MCl(g) f.lcmst"1) M+(g)+Ci-(g)
M + (g)+ er (g) FM(g)+ Cl(g)
(~ qccfufi<Ml)
('() a!tCill'Sf~il 'lll>ililM<tS ~: ~~ ~ ~ [~: NaN03, CaC03, KN03, Cu(N03)2l, '8
~ :~J~~l~lffi(l) ~~ I
('!") lftlill'Sf~il 'l~: cm~ ~~IC'il' ~~ ~ ~. '8mD° WI" (Watch glass),~~ I
1-0~tlliltJSIHl<ll
/tll~~
~
~~
"llllJfkl~: ~ "'l~IJ!il'!:I~Na+, Ca2+, K+ '<3 Cu2+ ~ -1-=il&<15~-=1 1
~) ~: ·~ ~ ~ ~ ~98f ~ ~ ~ HCI ~~ ~~~~ ~ ~
~ lfOO' ~ ~ <fi"@ ~~ ~ ~ 91~C<1'if'l'~~ ~ ~ <qSJfGl{:!"1 ""1"11'8'(1)'$1"1C(1) fi<Ml
~~I~~~~~~~~ <fi"@ ~ ~ (Ground state)
~~~~~<fi"@l~~~~~11~1f<l;qs~~~~f.if.l>ffi!i•
~ ~ ~~~ ~91_Cf ~~~(!)"@I ~iStt<f.~ 151"1<1'$1!i ~~ ~ ~·j ~
~~ ~ 91~C<1'if''t <fi"@ ~ <qSJfGl{:!"1 ~~~I~~~~~~~~
HCI ~~ ~~~ ~~ ~ C*l'$1~S ~ 91fuef'5 ~~I i515898f ~~ ~ ~98f lSMct ~ ~~
~;qsJfGl{:!C"1'$1 ~~~~9f~·~qccfufi'Mt ~~~~·~ ;qsJfGl{:!"1C(1) ~
~~I
I MZ + HCI (~)~MCI+ HZ[~~' M = ~~ ~ <j)Jt'Gl{:!"1, z = ~~ ~ 151]1"11{:!"1]
MCI~ MCl(g)
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-2-320.jpg)

![i) ~~ (NaCl, FeS04, v) ~ lll'ffi:>tl~l'i~S lx)~~~
FeCh) (K3[Fe(CN)6]) [(CH3COQ)2Pb] a<M
ii)~ 9flfir vi) ~ lll'~l:>tl~l'il~S x) C<tffi~I~ ~
(~[Fe(CN6)]) a<M .. [Ba(N03)2] a<M
iii)~~~ (HN03) vii)~~ (AgN03)a<f't
iv) 911'1Pt~I~ ~~Rk~lc-W viii) ~JIC~IM~I~ ~1~0!!:11<JIl~S
(K2H2Sb201) (NH40H)·~
(<lf) ~: a<M·'l:~ ~~ 00.QJT, ~ '<3 ~I a<fCct ~ ~ ~ ~ ~ ~ ~~~ ~
~ ~ ~ ~ ~ cqmr ~I ~ ~ ~·.~ ~ ~ <tSJtGl~'i ~~~ '3lJl'il~'i
~ ~ ~I ~ ~ ~ ~ <tSJiUl~'i ~~~ '3lJHlm:'i~ ~'i~ll!ll~ ~~ -!'.jl<tJl!>i~ ~~
<ID1 I ~ ~ ~l~'iC<tS ...1'il'8><tS~CCrn ~ .21rnliStifl~ ~. QTM ~ QJT ~ ~ ~ ~~~
f<t<tSl~C<tS~ '3lJl'il~C'i~ v:1~1l!ll~ ~~ (51"~ ~ ~~) 51"~ ~ ~Jl'il~'i '<3 f<t<tSl~C<tS~ <tSJiUl~C'i~
~'i~ll!ll~ 'G~ ~ -!'.jl<tJ!ll~ ~~ ~~~~ ~ ~ 51"~8N9f ~ QJT I ~ ~ ~.~ ~
~ ~ ~ ~ ~ 51"~8N9f ~9ijT QJT I 51"~8NC9Gf ~ ~ C<l'R'ZJ~"'l<tS '<!'cf 914'C<t"il'C'rn ~
. ~ ~ "1'il'8><tS~'i ~ ~ I
51"~8N9f=~ 'G~ >-!'.ll<tJ!ll~ 'G~
(~) ~c~llSfifl~ ~l:>tl~M~ ~:
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-5-320.jpg)
~
·m ~ <mf'(1S" 00 ~ KFe[Fe(CN)6}J:+2K+(aq)
C¥Rl:>tl~lij~15a<fct~fl I ('iftv ~ff 5fl:f8N9t) '
.. ~- . .
~) 5f98f l!l<i$ C"'lfil11l~Cl!l'I "'i~'tf'lij(ftt ~)~~~5fl:f8N9f~I ~) FeL+~~
2-3 fir.~. Fe2+~~a<fctm ~:
~ 2 - 3 ~ ~tfii1~111 Fe2aq) + K4[Fe(CN)6](aq)~
C¥C'Sll"'ll~lijl~15 a<fct ~fl I K2Fe[Fe(CN)6],l,+2K+(aq)
(~~ff 5fl:f8N?f)
[f.l~: l!l~ ~. K2FeII[F:e(CN)6]
~ ~ ~IN ~
~-
~~~~~~
KFem[Fe(CN)6] ~ Q3l" I ~ ~
~~~~~5{l:f~
~]
~I Fe3+~ .. ti11'6i<P'Sli1: ~ I <-l>) $. ~ ~ 5fl:fgc'lfi"9f91"CW I e 1<-l>)Fe3+~~
<-l>) l!l<i$ C"'lfit1l~Cl!l'I "'i~'tf'lij(ftt 2 - ~:
3 fir.~. Fe3+ ~ ~ a<fct Rm Fe3+(aq)+K3[Fe(CN)6](aq)~
~ 2 - 3 ~ ~tfi't~l11 Fe[Fe(CN)6],l,+ 3K+(aq)
C¥Rl:>tl~lijl~15 a<fct ~fl I ('ifV ~ 5fl:f8N9f)
~) 5f98f l!l<i$ C"'lfil1l~Cl!l'I ~ ~)$. ~ 5fl:f8N9f ~ I ~) Fe3+~~
~ ~ 2 - 3 fir.~. Fe3+~ ~:
~~Rrn2-3~~ Fe3+(aq)+~[Fe(CN)6](aq)~
C~C'Sll"'ll~lij~5 ~~fl I KFe[Fe(CN)6],l,+JK-f(aq)
('ifV ~ 5fl:f8N9f)
~8C'll'9fif: ~ ~·~ ~ ~ ~ ~.~>~ a<fct ~ C"'lfil1l~Cl!l'I ~ ~ ~ -
~fir.~. ~ a<fct ~ I 1£1~ 98f cajir ~ ~ ~ ~ ~ ~ ~ - ~ ~ .21C~liSt~~ ~
.~ fll ~ ~ ~t~J){G14> ~ 5fl:f8N9f 9fCW iWf 5fl:f8N~ ~ ~ ~ l!lr-1
"OI(ft! l"OIC<l 91( C<l"lf"t fl I -
(~) "1(c<q'*l''1~: ~~ "1iil'6i<P'Sli1:
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-6-320.jpg)
![ii<,
i '
I
(5)~:
i) ;qM•~Jl<!il<!il 9tCcf c&iGfi;S;q ~1CG'11~1c;q ~ ~ ~ ~ ~ c~ ~ ~ 1
ii)~~~~9flfif~~~~I
iii) >t<!il<q<!ill~'fl!> ~ ~ ~;q~ICI!>~ ~-~~~ill I
~)~~
l!l~~~~~
soi-~ ~ ~ oo '5IN
~ ~ ~ ~ ~- soi-(aq)+Ba(N03)i(aq)~
~ ~ I BaS04-!..+2 NO) (aq)
- (~f~~8N9f)
BaS04 s +HN03 dil -)-NOreaction
~) ~ ~ 5ltf8N9f 9fCW ~ ~
l!lM 15(!3<q'fur I
~:
QI<!')~ >tla>JC~l&<!il ~ ~tf8N9f QI<!') soi-~~
~ ~ ~ HNO) l!l ~;q'fur I
soi-(aq) + (CH3C00)2Pb(aq) ~
PbS04-!..+2CH3COO-(aq)
(~ 5li:f8N9f)
PbS04(s)+HN03(dil)-)-No reaction
Cl' I soi ·~tWP'liilW'll~:
<F)~l!I~~:
l!l~ C>tfit1ll~Cl!l'I ~ ~ ~
~ soi-~~~oo
~~~~l!l~~
~~L
2 cr(aq)+(CH3COO)iPb(aq)-)-
PbCii-!..+2CH3COO-(aq)
(~ 5ltf8N9f)
~)~'5l~8N9f~~~~ ~)Cr~$-,._.
~~I
~:
'()~I!!~
l!l<!$ C>tfit1l~Cl!l'I 91~"l'l"'!CG'! 2 ~ 3
1ir.~. er ~ ~ ~- oo
~~l!l~~~~I
8 I <!') ~ ~ 15llf~9f 9fCW I 8 I <!') er ~~
~tf~ ~ HN03 l!l 151!3;q'fur m
'<~NiRl'8'NRiOH l!l ~~I
~:
Cr(aq) + AgN03(~q) ~ AgC-l(s)-!..
- ··r--:q -~... -
+ NO)(aq) . f>t1~f~~~~). -
AgCl(s) + HN03 ~ NoReacti~n--_ .- ~'- · -
~:.- ;;:i,(' - -- ~
AgCl(s) + 2NH40H(aq) .
A H3)2]CIa +2H20
8 I Cl-~ "liil'64'llii:
<!') l!l~ C>tfit11l~Cl!l'I ~~ er
~ l!l~~~oo~~
~~~~~~I
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-7-320.jpg)
![oo~ tfl'Slt:
<f)1!'f~~: ~~c;'&>'Gffl~C<l >l'!l<l'!ll~<f~~™ 5-10Jl1g ~4Plir<f~C~liSTOll~ ~
9flR ~~~a<f9"~~ I
~) ~: ~ ~ ~ ~ ~ ~ ~ ~ ~ a<r9" ~ c>1Fil~l~Ci!!il ~ ~ ~ - ~
N.~.~a<rct~ I l!j~ 9Rf ~~ ~~~~ ~ ~ - ~ ~ ~C~liSTOll~ ~
~
9llR
~-~: ~ ~ 5!W1 ll'ji'jli8'<t>'!l"1
.!'.j<fCct 5!W1 ll'ji'jli8'<1S'!l"1 <r'!1l ~I
5T~gN9f=~ ~™>.!'.jl<lJl!>l'!l ~™
(~) f!C4111Sf0ll41 '511"1141M<l5 ~:
~ "!<l'ct [CaCii, ZnS04, A!Cb, CuS04, (NH4)iC03], ~ 9ftf.21, GIJIC):llM~l):l 51'l!IC"1U a<rct,
~~- l!l~, ~l~JJJal(J)IRl<P l!l~; G!Jfc):lfFBH~ ~. ~ C~C'!ll>11~1"11~5 a<rct, C>l)Nl~l):l
~l~C&il'l!l~5 a<rct, ~ a<rct, ~ G!IUllSl~5 a<rct, ~l~C&11Ci51"1 >11"1~1~5 ~I
(<IS)~: a<rct "i'ffl" ~~ 00.~.~IS~ I~~~~~~~ l!l~ ~
~ ~ ~ ~ $Cf<I> "lM ~I ~ ~ ~ ~ ~. ™ 41JIUl~"1 l!l<f~ 5111"11~"1
~ ~ ~I ~ ~ ~ ~ <tJtUl~"1 l!l<f~ GIJl"11~C"1'!1 "1"1~1!ll'!l ~~ .!'.!l<ll~l'!l ~™
~I ~ -~ G!l~"1C<tl "'1"1li8'41'!1C'Rl ~ ·~C~liSTOll~ ~ ~ ~ ~ ~ ~ ~ l!l~
M<tll'!lC<tl'!l GIJl"11~C"1'!1 ~~ ~™('5T~ ~ ~™) '5T'<l<rt ~ 51)1"11~"1 IS f.1<tSl'!1C<tl'!l <tJIUM"1'!1
"1"1~1!ll'!l ~™ ~ .!'.jl<lJ~l'!l ~™ 151N>'i!!i~ *@' ~ 5T~fgN9f~ ~I ~ ~ ~ ~ ~
~ ~ ~ ~ ~ 5T~~9f ~91:f~I 5T~g~9Rf·l!l ~ C4R~J~"l<t> <f'f ~ ~
11
I
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-8-320.jpg)
![2+2H20
. )
Na2Zn02+2H2S~ZnS-l.+Na2S+2H20
(~~~8N9t)
·~rzn + ·.•
~~I
I Zn2+~~: ~J(l))~~~gN9f~I
~~Xt.~ ~:
2 - 3 fir.¥!'. 2Zn2+(aq)+K4[Fe(CN)6kaq)~
n2+~ ~ ~ Zn2[Fe(CN)6].J..+4K+(aq) __ .. -·
m ~ 2 - 3 ~ c~~~n9t)' -- --------·--
~ Ctj>C~IJil{ll<ifa:15 ---
~~~I
CaC03 .J, +2NH4 (aq)
(~~~gN9f)
~) Ca2+ ~I~) Q~ . ·~ 2 - 3 ~)~~~gN9f~I
fir.¥!'. Ca2+ ~ ~ ~:
~ Rm ~ ~ Ca2+(aq)+(NH4)zC03(aq)~
~ 151JIC111H{ll11
~a<fCt~~·I
coo . .. COOH
I /Ca+2HCI ~I +CaCl2
COO . COOH
GIJIC11 lf.hll11
~~~I
~ I (J)) Ca2+
~~I
~I (J)) Zn+
~~I
COONH4 coo
2+
I i + ·Ca (aq) + . (aq)~ Ca + 2NHiaq)
COONH4·' coo/ i:
~~if!t'll'91
ca2+~ ~ ~
m~2-3~
Gl~l(}'l'G
~ ~I ~ ~ ~ <ref ~ ~$:m~9t ~ ~ ~~gN~ ~ ~ ~ Q~
~l<Ali::?IC<I ~~I
·--~-
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-9-320.jpg)
![<2" I ~Jl~C"'ll 11l~f.l'iS~<P ~IC{ll514:15 I!!~
15fl:f8N9f 911'8m ~ I
~:
2NH! (aq)+2KOH~2NH3+2!5-:+2H2.0
2K2[Hgl4]~4K.1+2Hgl2 / ,, ,
2Hgl2~ Hg214 .,,.,.- .
Hg214+2NH; ~NH2[Hg2h]+NH4I
/ _. (~~)
<ti NH:~~:
l!l~m~
~OfCO'f 2 - 3 fit.~.
NH!~~
00~3-4~
~~~~I
~) Cu2li I!!~ ~ 15fl:f8N9f 91WC<f, ~ Ii I!!~ ~ ~) Cu2+"(S~ I
~~I .,
Cu+8 I ~)
~~I
8 I ~) ~~ C'l'C~l"'11WH~Cl5~ ~ ~ ff
15fl:fgc'lf9f ~ I
~:
Cu2+(aq)+~[Fe(CN)6](aq)~
Cu2[Fe(CN)6]..l..+4K+(aq)
(~ ~ 15fl:f8N9f)
~I
81Cu2+~
~)I!!~ m ~
~ OfCO'f 2 - 3
fit.~. Cu2+ ~ ~
a<f't m ~·I - 2
~ 91SIPHJ 111
a<f't
NaAl02+NH4CI~Al(OH)3..l.. +NaCl(aq)+NH3(g)
(~~)
~I
~)(~)~~~~ ~ )~~~AI(OH}i~~oo~1
NH4CI ~~~~ ~:
~)Al +~~I
Al3+~ I ~) ~ ~ oURf~ 15fl:f8N9f ~ ~ <6lR>~'@ ~ I ~)
NaOHl!l~~~~I ~~I
~:
Al3+-t3NaOH(aq)~Al(OH)3J..+3Na+
(~~)
Al(OH)3(s)+NaOH~NaAl02+2H20
(~)
~I
~IAl3+~~:
~) 1!1<t$ ~ ~
~~ 2-3 fit.~.
At3+~~~
m~~ 1-2
~ 9 .91@ ~R>Rl'@
NaOH I!!~~~
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-10-320.jpg)
![i
I l
(o)~:
i) •U<IQ;IC'!l~ ~ c'G$Gffl©;q 'OICOil'OIC<l ~ ~ ~ 9flf.t~ c~ ~ ~ I
ii)~~~~9flf.t~~~~I
iii) >t~<l~l~"f!> ~ C<lSt-1151<1~1C~~~~~i{t I
coj-~~~
m ~ 2 - 3 ~ coj: (aq)+Ba(N03)2(aq)~BaC03(s)-l.+2NO]
Ba(N03)2 I!!~ ~ BaC03(s)+2HCl(aq)~BaC]i(aq)+C02(aq)t+H20(1)
~~I
. - ~ -.,_
~·;
~) 1!1<1$ C>tfiMl:Wl'I
91m..,.101c., 2 - 3 N.~.
·- .
~ ~ 'Ol.,'OIC<I <rqt
~1!1~~91::f~
~ 9ltfiRf~·~
~I
~l<fr)~~~~~~~l!j~
~91:l' ~i.~. 9tlf.Br.-~ ~ ~ ~ 9flf.t
~~I .
~:
co~-(aq) + 2HCI(aq)~ co2(g) +820:+ 2c1- ·
<;a(OH)2(aq) + C02---+ CaC03(~)·l~H20 .
(~~)
0 t'.'" ..r: ~-c.... -c: ....,.
~ 1<fr) co;-llll1t1c01<s1
~:1!1<1$~~
~ ~ coj-
~~m~
5 - 7 ~ ~ HCI
~·~~~~
~~~wt'~~
~ qBft ~ .~
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/labmanualhelpingbanglandc-180108113306/85/Salt-Analysis-in-Bangla-MANIK-11-320.jpg)
