SAFC Customer Poster - PEACe Conference 2015
•
1 like•333 views
Utilizing Cell Imaging Technology to Shorten Timelines and Reduce Development Resources by Earlier Identification of High Producing Recombinant CHO Cell Lines
Report
Share
Report
Share
Download to read offline
Recommended
US Pharma presentation on clone screen strategy for monoclonality using Solen...

Presentation by a Cell Metric CLD customer in US about their use of the system in clone screening for cell line development.
This presentation is made available courtesy of Momenta Pharmaceuticals and IBC Conferences
Fujifilm Diosynth poster from 13th PEACe Conference, Valencia, Spain

Assurance of Monoclonality of Recombinant CHO Cell Lines Using High-Resolution Imaging
ESACT 2017 Poster - A new cell line development platform for sigh efficiency ...

Initial data presented on the new VIPS single cell seeding technology was presented in the prestigious ESACT poster sessions in Lausanne by Dr Ian Taylor (15-17th May, 2017)
Thomas Sterovsky - Polymun Scientific (Vienna Conference, April 2016)

Monoclonality: Challenge or Chance?
CASSS Forum Poster - Enabling robist cloning method qualification in cell lin...

Use of fluorescence on Cell Metric CLD to specifically qualify your cloning method.
Poster presentation - Documented Clonality Report for Regulatory Submission

Poster presented at CCE XV, May 2016
Arna Andrews, CSL limited (vienna, april 2016)

Use of imaging to demonstrate clonality of production CHO cell lines
Clonality report for cell metric customer launch

New clonality reporting for Solentim Cell Metric; facilitates documentation of clones for regulatory reporting process
Recommended
US Pharma presentation on clone screen strategy for monoclonality using Solen...

Presentation by a Cell Metric CLD customer in US about their use of the system in clone screening for cell line development.
This presentation is made available courtesy of Momenta Pharmaceuticals and IBC Conferences
Fujifilm Diosynth poster from 13th PEACe Conference, Valencia, Spain

Assurance of Monoclonality of Recombinant CHO Cell Lines Using High-Resolution Imaging
ESACT 2017 Poster - A new cell line development platform for sigh efficiency ...

Initial data presented on the new VIPS single cell seeding technology was presented in the prestigious ESACT poster sessions in Lausanne by Dr Ian Taylor (15-17th May, 2017)
Thomas Sterovsky - Polymun Scientific (Vienna Conference, April 2016)

Monoclonality: Challenge or Chance?
CASSS Forum Poster - Enabling robist cloning method qualification in cell lin...

Use of fluorescence on Cell Metric CLD to specifically qualify your cloning method.
Poster presentation - Documented Clonality Report for Regulatory Submission

Poster presented at CCE XV, May 2016
Arna Andrews, CSL limited (vienna, april 2016)

Use of imaging to demonstrate clonality of production CHO cell lines
Clonality report for cell metric customer launch

New clonality reporting for Solentim Cell Metric; facilitates documentation of clones for regulatory reporting process
Presentation by Systimunne - at the Cell Line Development & Engineering Summi...

Reducing the timeline to produce a monoclonal cell line expressing a clonical candidate bispecific antibody
Valitacell Technology

Learn more about the Valitacell fluorescent polarisation based IgG quantification assay 'ValitaTITER' and about our novel ChemStress fingerprinting assay for cell line development. For more information about our products and pricing, please contact info@valitacell.com
Joel welch.pdf (amsterdam, april 2017)

Latest guidance from the US FDA o the importance of clonality for mammalian cell banks. This was presented at the Cell Line Development Conference in Amsterdam, 24th April, 2017
Rachel novak (casss forum, wahington dc, jan 2017)

Regulatory Perspective on the Evaluation of Clonality of Mammalian Cell Banks
Development of novel chemically defined media for CHO cell applications

Development of novel chemically defined media for CHO cell applicationsNational Institute of Biologics
novel chemically defined media for CHO cell applicationsIn Vitro Alternatives in Toxicology

Historically, genetic toxicology has been comprised of bacterial and cell based in vitro assays such as the Ames assay (a bacterial mutagenicity assay), Micronucleus and Chromosomal Aberration assays (mammalian cytogenetic assays), and Mouse Lymphoma Assay (in vitro mammalian cell gene mutation assay). These were routinely used for safety evaluation and are still part of the standard core battery. The emergence of new technologies has facilitated the development of in vitro methods for safe and effective drug and chemical testing.
This BioReliance® toxicology services webinar will explore alternative models, including 3D skin models that comply with the EC Scientific Committee on Consumer Safety (SCCS) recommendations. It will also discuss how the 3Rs (Replace, Reduce, Refine) Principle advocates the exploration of such alternative methods while achieving required goals.
In this webinar, you will learn:
• About in vitro alternatives to animal toxicity testing in pharma, chemical, tobacco, and personal care products.
• How the 3Rs (Replace, Reduce, Refine) Principle advocates exploring alternative methods without compromising the required goals.
• Alternatives to comply with the 7th Amendment to the EC Cosmetics Directive.
Development of quality control assays for cell-based medicinal products (ISCT...

Development of quality control assays for cell-based medicinal products (ISCT...Quality Assistance s.a.
Dr. Fabian Vandermeers from Quality Assistance spoke on Development of quality control assays for cell-based medicinal products at ISCT 2017 in London.
For more information on this topic, visit: http://www.quality-assistance.com/analytical-services/CBMPs
For more information on our expertise and services, visit: www.quality-assistance.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/quality-assistance
Twitter: https://twitter.com/QA_Belgium
Facebook: https://www.facebook.com/QualityAssistanceBelgium
Google +: https://plus.google.com/103676189647965359292
Quality Assistance S.A. is a leading European Contract Research Organisation providing the pharmaceutical industry with all the analytical services required by EMA and FDA regulations for the development and marketing of innovative human medicinal products.
We assist our clients from candidate selection, through non-clinical and clinical studies, to marketing authorisation, using our state-of-the-art, product-dedicated expertise in analytical sciences.
For each customer and each project, we design customised solutions, define analytical protocols, develop and validate specific new analytical methods and perform characterisation, stability, pharmacokinetic, biomarker and immunogenicity studies as well as batch release testing, in order to evaluate the Quality, Safety and Efficacy of the given drugs.The Comprehensive Guide to Genotoxicity Assessment

Discover solutions for all phases of product development for genetox assessment from in silico analysis, screening, mode of action assessment, or GLP regulatory required assays. Our BioReliance® Genetic Toxicology Services director will share specifics and rationale for each assay category.
In this webinar you will:
- Learn the required regulatory assays
- Understand why each assay is used and how to employ different assay designs
- Learn different assays and techniques to screen potential compounds and understand mechanism and mode of action
Presented by Rohan Kulkarni, Ph.D., ERT, Director Toxicology, Study Management on February 9, 2017
Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)

Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)Verhaert Masters in Innovation
Cell based therapy is the most promising innovation in medical. The biggest challenge? It’s a complex, extensive and manual production which results in a small scale and expensive treatment. We’ll dive in the technical challenges to automate and scale cell production and how cell transfection can be stimulated by the technology transfer of optics.WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...

WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...Quality Assistance s.a.
Valérie DEFFONTAINE, R&D Scientist, Quality Assistance
Webinar held on 8th June 2017.
The discovery of human pluripotent stem cells 10 years ago turned the spotlight on the potential of pluripotent stem cells for personalised cell therapy. The scientific interest then quickly shifted towards the use of these cells for safety pharmacology, drug discovery and disease modelling. For all these purposes, in the mid to long term, properly characterised cell banks will be necessary.
The characterisation of embryonic (ESC) and induced pluripotent stem cells (IPSC) used for manufacturing requires the development and validation of analytical methods (e.g. flow cytometry, microscopy, QPCR and bioassays). Cell characterisation includes the testing of cell product identity, determination of impurities, and assessment of biological activity and viability. Among the techniques available, flow cytometry is widely used to assess the expression of cell markers. Our laboratory has developed flow cytometry panels dedicated to the characterisation of extracellular and intracellular markers of ESC and IPSC, and to the detection of cell-related impurities. We proposed a method for the validation of flow cytometry panels according to the recommendations of international guidelines on the validation of analytical methods.
IPSC differentiated into cardiomyocytes and MSC-like cells were also used to test the performance of our flow cytometry panels to accurately monitor the manufacturing process of cell products.
In addition to the technical tips, this webinar aims at presenting a critical view on the use of flow cytometry platform for cell characterisation.
For more information, visit http://www.quality-assistance.com/analytical-services/CBMPs Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...

In this webinar, you will learn about:
- Risk assessment approaches in upstream process development
- How early cell line development stage is linked to subsequent steps in the bioprocess to assure the quality of the final product
- Benefits of having a completely chemically defined cell line development process
Detailed description:
Chinese Hamster Ovary (CHO) cells are the preferred host for producing biotherapeutics where cell line development (CLD) is the foundation of the bioprocess. CLD processes are expected to be robust while meeting a myriad of regulatory requirements. The choice of production cell line, culture conditions, and having a chemically defined (CD) CLD process by using CD cloning media can impact the subsequent measures for the CMC (Chemistry, manufacturing, and controls).
In this presentation, we will discuss these choices and their impacts on subsequent bioprocess and CMC testing required by regulations and the benefits of incorporating CD cloning media into the CHOZN® expression platform.
More Related Content
What's hot
Presentation by Systimunne - at the Cell Line Development & Engineering Summi...

Reducing the timeline to produce a monoclonal cell line expressing a clonical candidate bispecific antibody
Valitacell Technology

Learn more about the Valitacell fluorescent polarisation based IgG quantification assay 'ValitaTITER' and about our novel ChemStress fingerprinting assay for cell line development. For more information about our products and pricing, please contact info@valitacell.com
Joel welch.pdf (amsterdam, april 2017)

Latest guidance from the US FDA o the importance of clonality for mammalian cell banks. This was presented at the Cell Line Development Conference in Amsterdam, 24th April, 2017
Rachel novak (casss forum, wahington dc, jan 2017)

Regulatory Perspective on the Evaluation of Clonality of Mammalian Cell Banks
Development of novel chemically defined media for CHO cell applications

Development of novel chemically defined media for CHO cell applicationsNational Institute of Biologics
novel chemically defined media for CHO cell applicationsIn Vitro Alternatives in Toxicology

Historically, genetic toxicology has been comprised of bacterial and cell based in vitro assays such as the Ames assay (a bacterial mutagenicity assay), Micronucleus and Chromosomal Aberration assays (mammalian cytogenetic assays), and Mouse Lymphoma Assay (in vitro mammalian cell gene mutation assay). These were routinely used for safety evaluation and are still part of the standard core battery. The emergence of new technologies has facilitated the development of in vitro methods for safe and effective drug and chemical testing.
This BioReliance® toxicology services webinar will explore alternative models, including 3D skin models that comply with the EC Scientific Committee on Consumer Safety (SCCS) recommendations. It will also discuss how the 3Rs (Replace, Reduce, Refine) Principle advocates the exploration of such alternative methods while achieving required goals.
In this webinar, you will learn:
• About in vitro alternatives to animal toxicity testing in pharma, chemical, tobacco, and personal care products.
• How the 3Rs (Replace, Reduce, Refine) Principle advocates exploring alternative methods without compromising the required goals.
• Alternatives to comply with the 7th Amendment to the EC Cosmetics Directive.
Development of quality control assays for cell-based medicinal products (ISCT...

Development of quality control assays for cell-based medicinal products (ISCT...Quality Assistance s.a.
Dr. Fabian Vandermeers from Quality Assistance spoke on Development of quality control assays for cell-based medicinal products at ISCT 2017 in London.
For more information on this topic, visit: http://www.quality-assistance.com/analytical-services/CBMPs
For more information on our expertise and services, visit: www.quality-assistance.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/quality-assistance
Twitter: https://twitter.com/QA_Belgium
Facebook: https://www.facebook.com/QualityAssistanceBelgium
Google +: https://plus.google.com/103676189647965359292
Quality Assistance S.A. is a leading European Contract Research Organisation providing the pharmaceutical industry with all the analytical services required by EMA and FDA regulations for the development and marketing of innovative human medicinal products.
We assist our clients from candidate selection, through non-clinical and clinical studies, to marketing authorisation, using our state-of-the-art, product-dedicated expertise in analytical sciences.
For each customer and each project, we design customised solutions, define analytical protocols, develop and validate specific new analytical methods and perform characterisation, stability, pharmacokinetic, biomarker and immunogenicity studies as well as batch release testing, in order to evaluate the Quality, Safety and Efficacy of the given drugs.The Comprehensive Guide to Genotoxicity Assessment

Discover solutions for all phases of product development for genetox assessment from in silico analysis, screening, mode of action assessment, or GLP regulatory required assays. Our BioReliance® Genetic Toxicology Services director will share specifics and rationale for each assay category.
In this webinar you will:
- Learn the required regulatory assays
- Understand why each assay is used and how to employ different assay designs
- Learn different assays and techniques to screen potential compounds and understand mechanism and mode of action
Presented by Rohan Kulkarni, Ph.D., ERT, Director Toxicology, Study Management on February 9, 2017
Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)

Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)Verhaert Masters in Innovation
Cell based therapy is the most promising innovation in medical. The biggest challenge? It’s a complex, extensive and manual production which results in a small scale and expensive treatment. We’ll dive in the technical challenges to automate and scale cell production and how cell transfection can be stimulated by the technology transfer of optics.WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...

WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...Quality Assistance s.a.
Valérie DEFFONTAINE, R&D Scientist, Quality Assistance
Webinar held on 8th June 2017.
The discovery of human pluripotent stem cells 10 years ago turned the spotlight on the potential of pluripotent stem cells for personalised cell therapy. The scientific interest then quickly shifted towards the use of these cells for safety pharmacology, drug discovery and disease modelling. For all these purposes, in the mid to long term, properly characterised cell banks will be necessary.
The characterisation of embryonic (ESC) and induced pluripotent stem cells (IPSC) used for manufacturing requires the development and validation of analytical methods (e.g. flow cytometry, microscopy, QPCR and bioassays). Cell characterisation includes the testing of cell product identity, determination of impurities, and assessment of biological activity and viability. Among the techniques available, flow cytometry is widely used to assess the expression of cell markers. Our laboratory has developed flow cytometry panels dedicated to the characterisation of extracellular and intracellular markers of ESC and IPSC, and to the detection of cell-related impurities. We proposed a method for the validation of flow cytometry panels according to the recommendations of international guidelines on the validation of analytical methods.
IPSC differentiated into cardiomyocytes and MSC-like cells were also used to test the performance of our flow cytometry panels to accurately monitor the manufacturing process of cell products.
In addition to the technical tips, this webinar aims at presenting a critical view on the use of flow cytometry platform for cell characterisation.
For more information, visit http://www.quality-assistance.com/analytical-services/CBMPs What's hot (20)
Presentation by Systimunne - at the Cell Line Development & Engineering Summi...

Presentation by Systimunne - at the Cell Line Development & Engineering Summi...
Rachel novak (casss forum, wahington dc, jan 2017)

Rachel novak (casss forum, wahington dc, jan 2017)
Development of novel chemically defined media for CHO cell applications

Development of novel chemically defined media for CHO cell applications
Development of quality control assays for cell-based medicinal products (ISCT...

Development of quality control assays for cell-based medicinal products (ISCT...
The Comprehensive Guide to Genotoxicity Assessment

The Comprehensive Guide to Genotoxicity Assessment
Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)

Scaling production of cell based therapies (by Jan Calliauw & Thomas Degreef)
WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...

WEBINAR Characterisation of human pluripotent stem cells (ESCs and IPSC) and ...
Similar to SAFC Customer Poster - PEACe Conference 2015
Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...

In this webinar, you will learn about:
- Risk assessment approaches in upstream process development
- How early cell line development stage is linked to subsequent steps in the bioprocess to assure the quality of the final product
- Benefits of having a completely chemically defined cell line development process
Detailed description:
Chinese Hamster Ovary (CHO) cells are the preferred host for producing biotherapeutics where cell line development (CLD) is the foundation of the bioprocess. CLD processes are expected to be robust while meeting a myriad of regulatory requirements. The choice of production cell line, culture conditions, and having a chemically defined (CD) CLD process by using CD cloning media can impact the subsequent measures for the CMC (Chemistry, manufacturing, and controls).
In this presentation, we will discuss these choices and their impacts on subsequent bioprocess and CMC testing required by regulations and the benefits of incorporating CD cloning media into the CHOZN® expression platform.
Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...

In this webinar, you will learn about:
- Risk assessment approaches in upstream process development
- How early cell line development stage is linked to subsequent steps in the bioprocess to assure the quality of the final product
- Benefits of having a completely chemically defined cell line development process
Detailed description:
Chinese Hamster Ovary (CHO) cells are the preferred host for producing biotherapeutics where cell line development (CLD) is the foundation of the bioprocess. CLD processes are expected to be robust while meeting a myriad of regulatory requirements. The choice of production cell line, culture conditions, and having a chemically defined (CD) CLD process by using CD cloning media can impact the subsequent measures for the CMC (Chemistry, manufacturing, and controls).
In this presentation, we will discuss these choices and their impacts on subsequent bioprocess and CMC testing required by regulations and the benefits of incorporating CD cloning media into the CHOZN® expression platform.
Improved Platforms for CHO Cell Line Development 

Review of the integration of CHOZN and UCOE cell line development platforms, and the performance of the combined systems.
Process improvement delivered by a high efficiency, automated single cell clo...

Presented by Andrea Gough, Solentim at CCE XVI in Tampa, FL (May 2018)
Fully Characterized, Standardized Human Induced Pluripotent Stem Cell Line an...

In this webinar, experts present a standardized stem cell line and its differentiation into neural cells for disease modeling and assay development.
Reproducible research with human induced pluripotent stem cells (iPSCs) depends on thoroughly characterized and quality-controlled cell lines. In this webinar, Dr. Andrew Gaffney and Dr. Erin Knock from STEMCELL Technologies describe the generation of a standardized induced pluripotent stem cell (iPSC) line. Developed with the upcoming ISSCR Standards Initiative characterization guidelines in mind, this highly characterized line is karyotypically stable, demonstrates trilineage differentiation potential, and expresses undifferentiated cell markers. Further, STEMCELL has developed a highly pure, ready-to-use neural progenitor cell product expressing PAX6 and SOX1 over multiple passages.
Dr. Knock shows how these multipotent cells are suitable for customized downstream differentiation to various CNS cell types, such as forebrain neurons, midbrain neurons, and astrocytes. These progenitor cells are the ideal controls for standardizing downstream differentiation protocols, modeling diseases, and assay development.
Key Topics Include:
- Discover how STEMCELL’s induced pluripotent stem cell lines are derived and characterized
- Learn how to differentiate induced pluripotent stem cell lines into all three germ layers
- Explore the features of STEMCELL’s neural progenitor cell product
- Differentiate neural progenitor cells into a variety of neural cell types, including neurons and glia
Scalability of Cell Culture Processes in Single-use Bioreactors using Differe...

Niket Bubna, Cameron T. Phillips, Sigma S. Mostafa and AbhinavA. Shukla. KBI Biopharma, Durham, NC
253rd ACS National Meeting & Exposition
April 2-6, 2017 • San Francisco, CA
#acsSanFran • www.acs.org/SanFran2017
CC3TM:A Stable,Sterile Analog of Poly-D-Lysine that is Optimal for the Cultur...

Poly-D-Lysine (PDL) improves attachment and growth of certain fastidious cells. PDL surfaces cannot be considered formally sterile, because current sterilization methods would compromise cell adhesion and often require controlled storage conditions over the usual 1-2 year shelf life. To address these issues, a non-biological analog of PDL was investigated for function and stability in cell culture. This synthetic organic polymer, CC3TM, displays a high amine group density and positive charge in neutral media.
Bioengineering theory presentation 2.pptx

This is presentation about bioengineering theory its principles and future aspects featuring all the perspectives
Cell Line Development: Reducing timelines and increasing titres 

Cell line development: Reducing timelines and increasing titres by identification of host cell lines with improved characteristics. To develop a mammalian expression platform which rapidly leads to efficient, robust and high quality biomanufacturing processes
Interventional orthopedics foundation amniotic tissue products poster

Testing of amniotic tissues to discover their best use for interventional orthopedics.
SeedEZ 3D culture methods and protocols - total protein extraction

SeedEZ 3D cell culture methods and protocols – total protein extraction and quantification. 3D cell culturing conditions influence protein extraction from cells for downstream analysis. Depending on extraction buffer used, an amount of protein from the extracellular matrix (in addition to cell protein) may be extracted. For these reasons, setup suitable controls. Here, it is shown how to extract total protein from cells cultured in 3D in the SeedEZ under different culturing conditions and how to apply a protein assay to quantify it. Protein assay results with 3D brain co-culture models comprising primary cortical neurons and primary-harvested and one-time passaged mixed glia (astrocytes and microglia) seeded into (a) uncoated SeedEZ substrate, (b) Poly-D-Lysine coated SeedEZ substrate, and (c) seeded in 7.5 mg/ml protein suspension into uncoated SeedEZ are shown.
Similar to SAFC Customer Poster - PEACe Conference 2015 (20)
Biotechnology and gene expression profiling for mechanistic understanding of ...

Biotechnology and gene expression profiling for mechanistic understanding of ...
Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...

Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...
Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...

Risk Mitigation in Cell Line Development: Regulatory Considerations and Impac...
Process improvement delivered by a high efficiency, automated single cell clo...

Process improvement delivered by a high efficiency, automated single cell clo...
A putative mesenchymal stem cells population isolated from adult human testes

A putative mesenchymal stem cells population isolated from adult human testes
Fully Characterized, Standardized Human Induced Pluripotent Stem Cell Line an...

Fully Characterized, Standardized Human Induced Pluripotent Stem Cell Line an...
Scalability of Cell Culture Processes in Single-use Bioreactors using Differe...

Scalability of Cell Culture Processes in Single-use Bioreactors using Differe...
CC3TM:A Stable,Sterile Analog of Poly-D-Lysine that is Optimal for the Cultur...

CC3TM:A Stable,Sterile Analog of Poly-D-Lysine that is Optimal for the Cultur...
Biopharma musculoskeletal disorders_draft 6-30-2013 jm edits

Biopharma musculoskeletal disorders_draft 6-30-2013 jm edits
Cell Line Development: Reducing timelines and increasing titres 

Cell Line Development: Reducing timelines and increasing titres
Interventional orthopedics foundation amniotic tissue products poster

Interventional orthopedics foundation amniotic tissue products poster
SeedEZ 3D culture methods and protocols - total protein extraction

SeedEZ 3D culture methods and protocols - total protein extraction
Recently uploaded
(May 29th, 2024) Advancements in Intravital Microscopy- Insights for Preclini...

(May 29th, 2024) Advancements in Intravital Microscopy- Insights for Preclini...Scintica Instrumentation
Intravital microscopy (IVM) is a powerful tool utilized to study cellular behavior over time and space in vivo. Much of our understanding of cell biology has been accomplished using various in vitro and ex vivo methods; however, these studies do not necessarily reflect the natural dynamics of biological processes. Unlike traditional cell culture or fixed tissue imaging, IVM allows for the ultra-fast high-resolution imaging of cellular processes over time and space and were studied in its natural environment. Real-time visualization of biological processes in the context of an intact organism helps maintain physiological relevance and provide insights into the progression of disease, response to treatments or developmental processes.
In this webinar we give an overview of advanced applications of the IVM system in preclinical research. IVIM technology is a provider of all-in-one intravital microscopy systems and solutions optimized for in vivo imaging of live animal models at sub-micron resolution. The system’s unique features and user-friendly software enables researchers to probe fast dynamic biological processes such as immune cell tracking, cell-cell interaction as well as vascularization and tumor metastasis with exceptional detail. This webinar will also give an overview of IVM being utilized in drug development, offering a view into the intricate interaction between drugs/nanoparticles and tissues in vivo and allows for the evaluation of therapeutic intervention in a variety of tissues and organs. This interdisciplinary collaboration continues to drive the advancements of novel therapeutic strategies.
Cancer cell metabolism: special Reference to Lactate Pathway

Normal Cell Metabolism:
Cellular respiration describes the series of steps that cells use to break down sugar and other chemicals to get the energy we need to function.
Energy is stored in the bonds of glucose and when glucose is broken down, much of that energy is released.
Cell utilize energy in the form of ATP.
The first step of respiration is called glycolysis. In a series of steps, glycolysis breaks glucose into two smaller molecules - a chemical called pyruvate. A small amount of ATP is formed during this process.
Most healthy cells continue the breakdown in a second process, called the Kreb's cycle. The Kreb's cycle allows cells to “burn” the pyruvates made in glycolysis to get more ATP.
The last step in the breakdown of glucose is called oxidative phosphorylation (Ox-Phos).
It takes place in specialized cell structures called mitochondria. This process produces a large amount of ATP. Importantly, cells need oxygen to complete oxidative phosphorylation.
If a cell completes only glycolysis, only 2 molecules of ATP are made per glucose. However, if the cell completes the entire respiration process (glycolysis - Kreb's - oxidative phosphorylation), about 36 molecules of ATP are created, giving it much more energy to use.
IN CANCER CELL:
Unlike healthy cells that "burn" the entire molecule of sugar to capture a large amount of energy as ATP, cancer cells are wasteful.
Cancer cells only partially break down sugar molecules. They overuse the first step of respiration, glycolysis. They frequently do not complete the second step, oxidative phosphorylation.
This results in only 2 molecules of ATP per each glucose molecule instead of the 36 or so ATPs healthy cells gain. As a result, cancer cells need to use a lot more sugar molecules to get enough energy to survive.
Unlike healthy cells that "burn" the entire molecule of sugar to capture a large amount of energy as ATP, cancer cells are wasteful.
Cancer cells only partially break down sugar molecules. They overuse the first step of respiration, glycolysis. They frequently do not complete the second step, oxidative phosphorylation.
This results in only 2 molecules of ATP per each glucose molecule instead of the 36 or so ATPs healthy cells gain. As a result, cancer cells need to use a lot more sugar molecules to get enough energy to survive.
introduction to WARBERG PHENOMENA:
WARBURG EFFECT Usually, cancer cells are highly glycolytic (glucose addiction) and take up more glucose than do normal cells from outside.
Otto Heinrich Warburg (; 8 October 1883 – 1 August 1970) In 1931 was awarded the Nobel Prize in Physiology for his "discovery of the nature and mode of action of the respiratory enzyme.
WARNBURG EFFECT : cancer cells under aerobic (well-oxygenated) conditions to metabolize glucose to lactate (aerobic glycolysis) is known as the Warburg effect. Warburg made the observation that tumor slices consume glucose and secrete lactate at a higher rate than normal tissues.
SCHIZOPHRENIA Disorder/ Brain Disorder.pdf

This pdf is about the Schizophrenia.
For more details visit on YouTube; @SELF-EXPLANATORY;
https://www.youtube.com/channel/UCAiarMZDNhe1A3Rnpr_WkzA/videos
Thanks...!
extra-chromosomal-inheritance[1].pptx.pdfpdf![extra-chromosomal-inheritance[1].pptx.pdfpdf](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![extra-chromosomal-inheritance[1].pptx.pdfpdf](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Slide 1: Title Slide
Extrachromosomal Inheritance
Slide 2: Introduction to Extrachromosomal Inheritance
Definition: Extrachromosomal inheritance refers to the transmission of genetic material that is not found within the nucleus.
Key Components: Involves genes located in mitochondria, chloroplasts, and plasmids.
Slide 3: Mitochondrial Inheritance
Mitochondria: Organelles responsible for energy production.
Mitochondrial DNA (mtDNA): Circular DNA molecule found in mitochondria.
Inheritance Pattern: Maternally inherited, meaning it is passed from mothers to all their offspring.
Diseases: Examples include Leber’s hereditary optic neuropathy (LHON) and mitochondrial myopathy.
Slide 4: Chloroplast Inheritance
Chloroplasts: Organelles responsible for photosynthesis in plants.
Chloroplast DNA (cpDNA): Circular DNA molecule found in chloroplasts.
Inheritance Pattern: Often maternally inherited in most plants, but can vary in some species.
Examples: Variegation in plants, where leaf color patterns are determined by chloroplast DNA.
Slide 5: Plasmid Inheritance
Plasmids: Small, circular DNA molecules found in bacteria and some eukaryotes.
Features: Can carry antibiotic resistance genes and can be transferred between cells through processes like conjugation.
Significance: Important in biotechnology for gene cloning and genetic engineering.
Slide 6: Mechanisms of Extrachromosomal Inheritance
Non-Mendelian Patterns: Do not follow Mendel’s laws of inheritance.
Cytoplasmic Segregation: During cell division, organelles like mitochondria and chloroplasts are randomly distributed to daughter cells.
Heteroplasmy: Presence of more than one type of organellar genome within a cell, leading to variation in expression.
Slide 7: Examples of Extrachromosomal Inheritance
Four O’clock Plant (Mirabilis jalapa): Shows variegated leaves due to different cpDNA in leaf cells.
Petite Mutants in Yeast: Result from mutations in mitochondrial DNA affecting respiration.
Slide 8: Importance of Extrachromosomal Inheritance
Evolution: Provides insight into the evolution of eukaryotic cells.
Medicine: Understanding mitochondrial inheritance helps in diagnosing and treating mitochondrial diseases.
Agriculture: Chloroplast inheritance can be used in plant breeding and genetic modification.
Slide 9: Recent Research and Advances
Gene Editing: Techniques like CRISPR-Cas9 are being used to edit mitochondrial and chloroplast DNA.
Therapies: Development of mitochondrial replacement therapy (MRT) for preventing mitochondrial diseases.
Slide 10: Conclusion
Summary: Extrachromosomal inheritance involves the transmission of genetic material outside the nucleus and plays a crucial role in genetics, medicine, and biotechnology.
Future Directions: Continued research and technological advancements hold promise for new treatments and applications.
Slide 11: Questions and Discussion
Invite Audience: Open the floor for any questions or further discussion on the topic.
Unveiling the Energy Potential of Marshmallow Deposits.pdf

Unveiling the Energy Potential of Marshmallow Deposits: A Revolutionary
Breakthrough in Sustainable Energy Science
Comparative structure of adrenal gland in vertebrates

Adrenal gland comparative structures in vertebrates
Structures and textures of metamorphic rocks

It is useful for the Under Graduating students for easy understanding and it's useful for the exam preparations.
FAIR & AI Ready KGs for Explainable Predictions

The increased availability of biomedical data, particularly in the public domain, offers the opportunity to better understand human health and to develop effective therapeutics for a wide range of unmet medical needs. However, data scientists remain stymied by the fact that data remain hard to find and to productively reuse because data and their metadata i) are wholly inaccessible, ii) are in non-standard or incompatible representations, iii) do not conform to community standards, and iv) have unclear or highly restricted terms and conditions that preclude legitimate reuse. These limitations require a rethink on data can be made machine and AI-ready - the key motivation behind the FAIR Guiding Principles. Concurrently, while recent efforts have explored the use of deep learning to fuse disparate data into predictive models for a wide range of biomedical applications, these models often fail even when the correct answer is already known, and fail to explain individual predictions in terms that data scientists can appreciate. These limitations suggest that new methods to produce practical artificial intelligence are still needed.
In this talk, I will discuss our work in (1) building an integrative knowledge infrastructure to prepare FAIR and "AI-ready" data and services along with (2) neurosymbolic AI methods to improve the quality of predictions and to generate plausible explanations. Attention is given to standards, platforms, and methods to wrangle knowledge into simple, but effective semantic and latent representations, and to make these available into standards-compliant and discoverable interfaces that can be used in model building, validation, and explanation. Our work, and those of others in the field, creates a baseline for building trustworthy and easy to deploy AI models in biomedicine.
Bio
Dr. Michel Dumontier is the Distinguished Professor of Data Science at Maastricht University, founder and executive director of the Institute of Data Science, and co-founder of the FAIR (Findable, Accessible, Interoperable and Reusable) data principles. His research explores socio-technological approaches for responsible discovery science, which includes collaborative multi-modal knowledge graphs, privacy-preserving distributed data mining, and AI methods for drug discovery and personalized medicine. His work is supported through the Dutch National Research Agenda, the Netherlands Organisation for Scientific Research, Horizon Europe, the European Open Science Cloud, the US National Institutes of Health, and a Marie-Curie Innovative Training Network. He is the editor-in-chief for the journal Data Science and is internationally recognized for his contributions in bioinformatics, biomedical informatics, and semantic technologies including ontologies and linked data.
insect taxonomy importance systematics and classification

documents provide information about insect classification and taxonomy of insect
Recently uploaded (20)
(May 29th, 2024) Advancements in Intravital Microscopy- Insights for Preclini...

(May 29th, 2024) Advancements in Intravital Microscopy- Insights for Preclini...
Cancer cell metabolism: special Reference to Lactate Pathway

Cancer cell metabolism: special Reference to Lactate Pathway
platelets- lifespan -Clot retraction-disorders.pptx

platelets- lifespan -Clot retraction-disorders.pptx
Unveiling the Energy Potential of Marshmallow Deposits.pdf

Unveiling the Energy Potential of Marshmallow Deposits.pdf
PRESENTATION ABOUT PRINCIPLE OF COSMATIC EVALUATION

PRESENTATION ABOUT PRINCIPLE OF COSMATIC EVALUATION
Comparative structure of adrenal gland in vertebrates

Comparative structure of adrenal gland in vertebrates
Circulatory system_ Laplace law. Ohms law.reynaults law,baro-chemo-receptors-...

Circulatory system_ Laplace law. Ohms law.reynaults law,baro-chemo-receptors-...
insect taxonomy importance systematics and classification

insect taxonomy importance systematics and classification
SAFC Customer Poster - PEACe Conference 2015
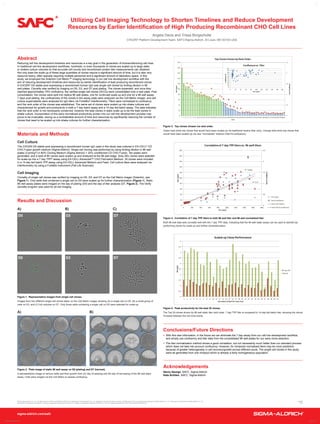
- 1. Utilizing Cell Imaging Technology to Shorten Timelines and Reduce Development Resources by Earlier Identification of High Producing Recombinant CHO Cell Lines Angela Davis and Trissa Borgschulte CHOZN® Platform Development Team, SAFC/Sigma-Aldrich, St Louis, MO 63103 USA Abstract Reducing cell line development timelines and resources is a key goal in the generation of biomanufacturing cell lines. In traditional cell line development workflows, hundreds, or even thousands of clones are scaled up to large static or shaken culture volumes so that accurate cell counts and recombinant protein titer measurements can obtained. Not only does the scale up of these large quantities of clones require a significant amount of time, but it is also very resource heavy, often typically requiring multiple personnel and a significant amount of laboratory space. In this study, we employed the Solentim Cell Metric™ imaging technology in our cell line development workflow with the aim of reducing development timelines and resources by earlier identification of lead producing recombinant clones. A CHOZN® GS stable pool expressing a recombinant human IgG was single cell cloned by limiting dilution in 96 well plates. Clonality was verified by imaging on D0, D3, and D7 post plating. The clones expanded, and once they reached approximately 75% confluency, the verified single cell clones (SCCs) were consolidated onto a new plate. Post consolidation, the clones were split into replica 96 well plates, one for continued scale up and one for a 96 well assay. 7 days post plating, the confluencies of the clones in the assay plate were analyzed via the Cell Metric imager, and cell culture supernatants were analyzed for IgG titers via ForteBio® interferometry. Titers were normalized to confluency and the rank order of the clones was established. The same set of clones were scaled up into shake cultures and characterized for growth and productivity in both a 7 day batch assay and a 14 day fed-batch assay. The data indicates that the rank order is not necessarily conserved, however the lead clones in static scale up to be the lead clones in shake culture. Incorporation of this early normalized productivity screen into our cell line development process may prove to be invaluable, saving us a considerable amount of time and resources by significantly reducing the number of clones that need to be scaled up into shake cultures for further characterization. Materials and Methods Cell Culture The CHOZN GS stable pool expressing a recombinant human IgG used in this study was cultured in EX-CELL® CD CHO Fusion growth medium (Sigma-Aldrich). Single cell cloning was performed by using limiting dilution in 96 well plates (Corning® ) in 80% Cloning Medium (Sigma-Aldrich) + 20% conditioned CD CHO Fusion. Ten plates were generated, and a total of 80 clones were scaled up and analyzed at the 96 well stage. Sixty (60) clones were selected for scale-up into a 7 day TPP® assay using EX-CELL Advanced™ CHO Fed-batch Medium. 30 clones were included in a 14-day fed batch TPP assay using EX-CELL Advanced Medium and Feed. Cell culture titers were analyzed via interferometry by using a ForteBio instrument (Pall Life Sciences). Cell Imaging Clonality of single cell clones was verified by imaging on D0, D3, and D7 on the Cell Metric imager (Solentim, see Figure 1). Only wells that contained a single cell on D0 were scaled up for further characterization (Figure 1). Static 96 well assay plates were imaged on the day of plating (D0) and the day of titer analysis (D7, Figure 2). The Verify clonality program was used for all cell imaging. Results and Discussion A) B) C) D0 D0 D3 D3 D7 D7 Figure 1. Representative images from single cell clones. Images from two different single cell clones taken on the Cell Metric imager showing (A) a single cell on D0, (B) a small group of cells on D3, and (C) full colonies on D7. Only those wells containing a single cell on D0 were selected for scale-up. B)A) Figure 2. Plate image of static 96 well assay on D0 (plating) and D7 (harvest). A representative image of various wells and their growth from (A) day of seeding and (B) day of harvesting of the 96 well static assay. Cells were imaged via the Cell Metric to assess confluency. sigma-aldrich.com/safc Figure 3. Top clones chosen via rank order. Green bars show top clones that would have been scaled up via traditional means (titer only). Orange dots show top clones that would have been scaled up via new “normalized” method (Titer/%Confluence). Figure 4. Correlation of 7 day TPP titers to both 96 well titer and 96 well normalized titer. Both 96 well data sets correlate well with the 7 day TPP data, indicating that the 96 well static assay can be used to identify top performing clones for scale up and further characterization. Figure 5. Peak productivity for the lead 30 clones. The Top 30 clones shown by 96 well static titer rank order. 7 day TPP titer is compared to 14-day fed-batch titer, showing the robust increase between the two time points. Conclusions/Future Directions • With this new information, in the future we can eliminate the 7 day assay from our cell line development workflow, and simply use confluency and titer data from the consolidated 96 well plates for our early clone selection. • The titer normalization method shows a good correlation, but not necessarily much better than our standard process which does not take into account confluency. However, for minipools normalized titers may be more predictive because of greater heterogeneity in cell recovery/growth across different pools. The single cell clones in this study were all generated from one minipool which is already a fairly homogeneous population. Acknowledgements Henry George, SAFC, Sigma-Aldrich Kate Achtien, SAFC, Sigma-Aldrich sh8956 1095 ©2015 Sigma-Aldrich Co. LLC. All rights reserved. SAFC and SIGMA-ALDRICH are trademarks of Sigma-Aldrich Co. LLC, registered in the US and other countries. CHOZN and EX-CELL are registered trademarks of Sigma-Aldrich Co. LLC. Advanced is a trademark of Sigma-Aldrich Co. LLC. Cell Metric is a trademark of Solentim. Corning is a registered trademark of Corning, Inc. ForteBio is a registered trademark of Pall Corp. TPP is a registered trademark of TPP Techno Plastic Products AG. sh8956_Poster for Peace Conference.indd 1 9/11/15 8:15 AM