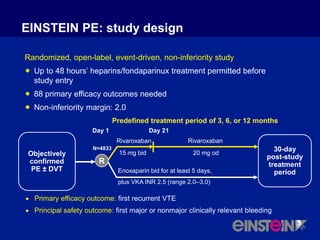
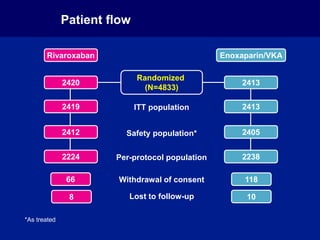
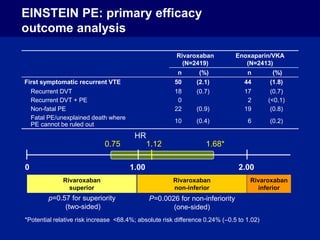
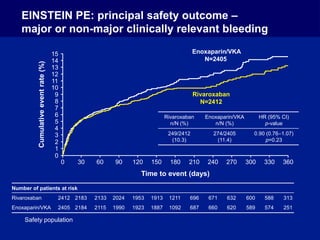
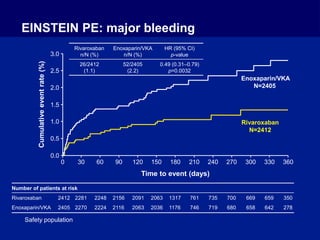
The EINSTEIN PE study evaluated oral rivaroxaban for treating symptomatic pulmonary embolism, demonstrating its non-inferiority to enoxaparin/VKA in efficacy with similar safety outcomes. Rivaroxaban showed significant superiority in reducing major bleeding events compared to enoxaparin/VKA. Overall, rivaroxaban's results were consistent across various patient demographics without indications of liver toxicity.