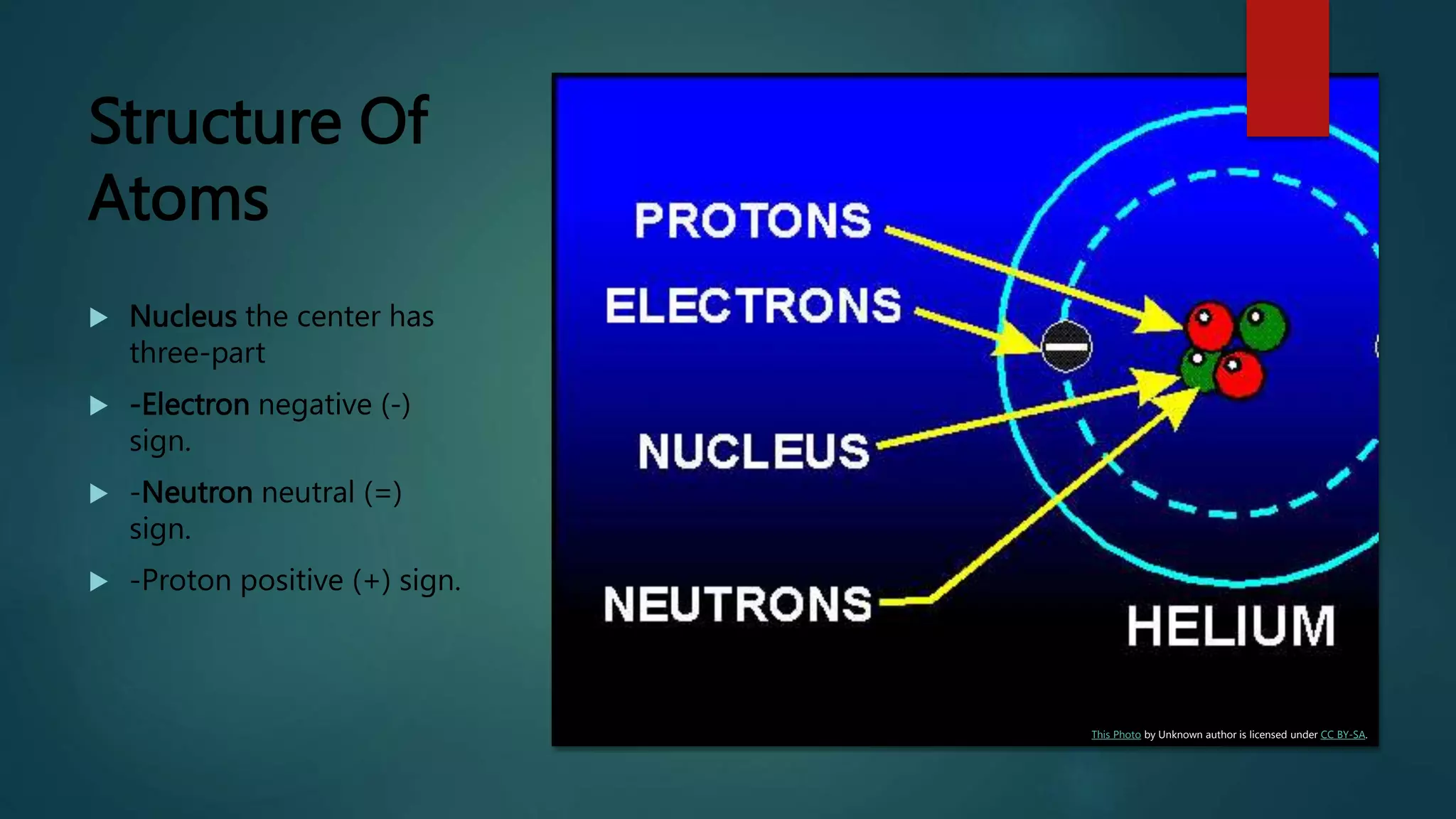
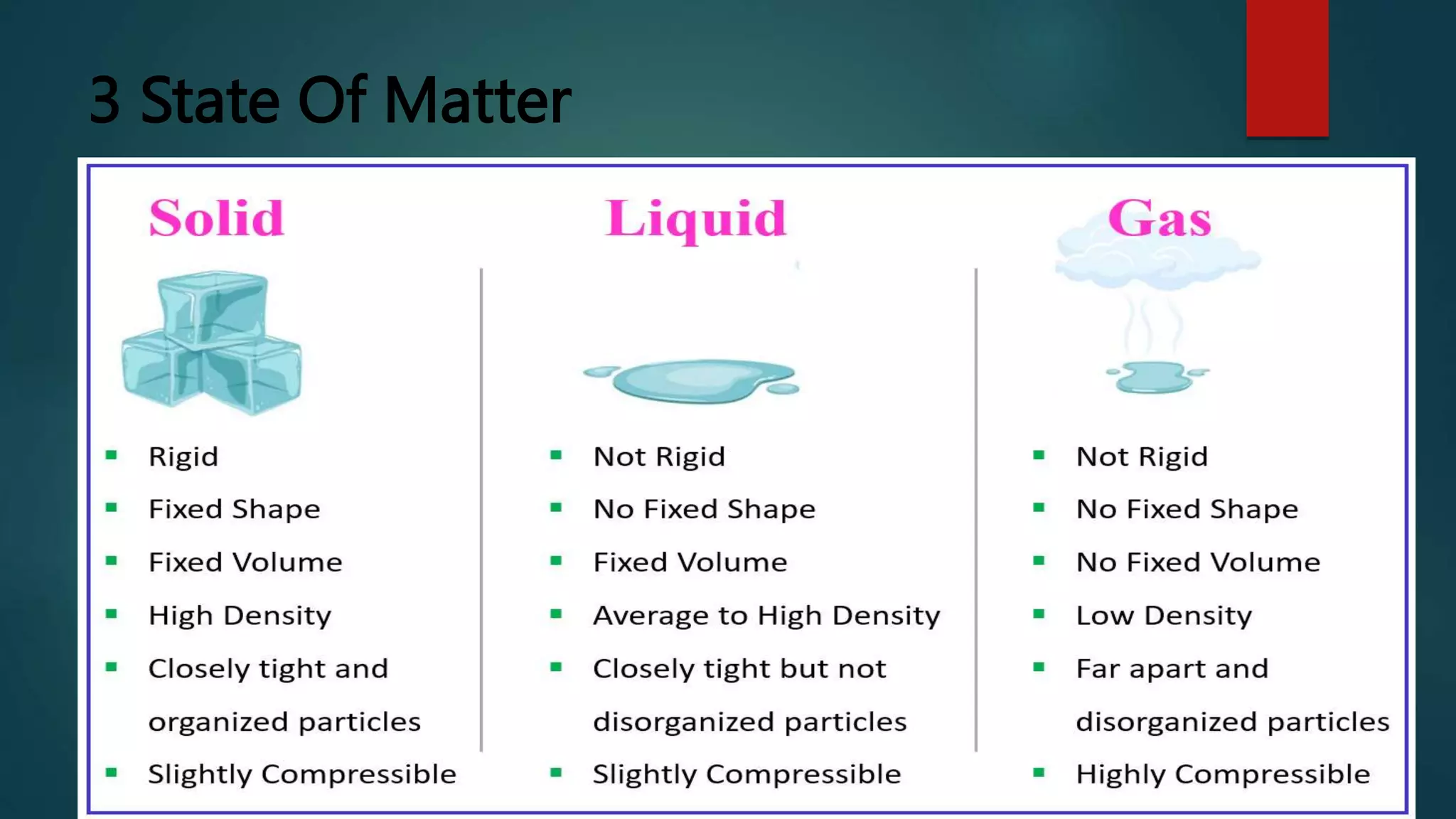
The document provides an overview of atomic structure, including the components of atoms (electrons, neutrons, and protons) and their significance in understanding matter. It discusses the properties and classifications of matter, emphasizing the three states (solid, liquid, gas) and the differences between pure substances and mixtures. Additionally, the document highlights various physical and chemical properties, phase changes, and the concept of diffusion.