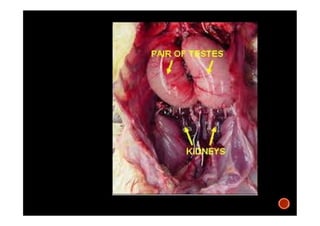
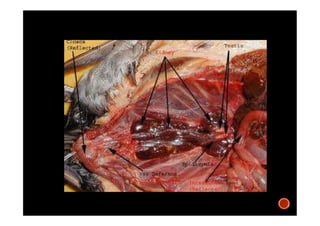
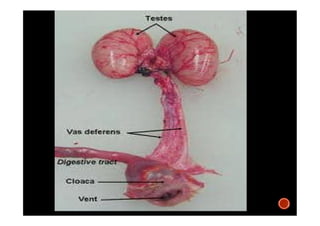
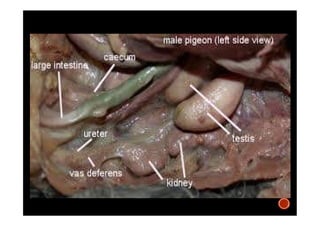
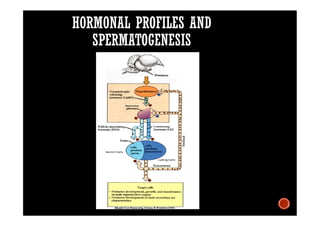
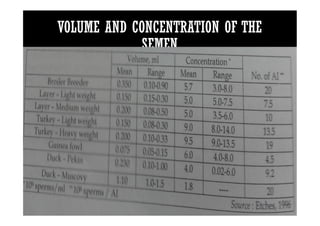
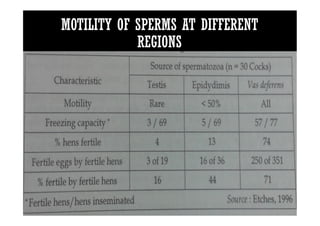
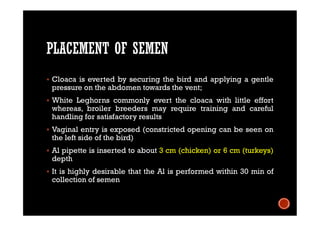
The document summarizes the avian reproductive system. It describes the major components including the testes, epididymis, ductus deferens, and papilla. It discusses spermatogenesis, semen production and transport, and the roles of hormones like testosterone. The timing of artificial insemination and techniques like semen collection, evaluation, and storage are also outlined. Caponization is described as the surgical removal of testes in male chickens to improve meat quality.