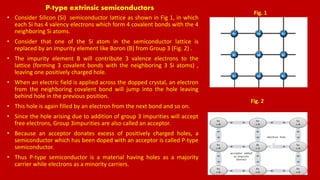
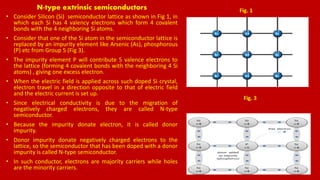
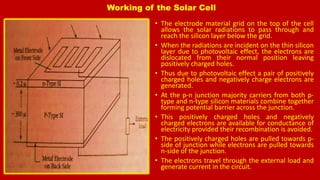
The document discusses renewable energy sources, particularly solar energy and hydrogen, highlighting their advantages over non-renewable sources. It also explains the principles of semiconductors, conductors, and insulators, detailing how p-type and n-type semiconductors function. Additionally, the workings of solar cells and the process of hydrogen production through electrolysis are described, emphasizing their potential as clean and sustainable energy solutions.