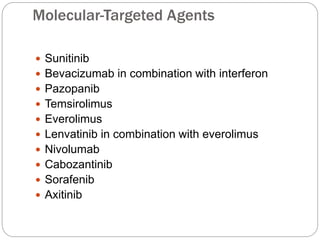
Three doctors presented on renal cell carcinoma. Some key points:
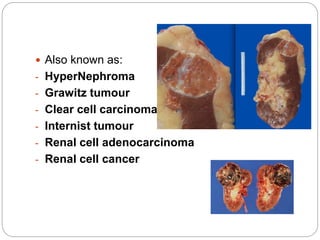
- Renal cell carcinoma arises from renal tubular cells and is the most common type of kidney cancer.
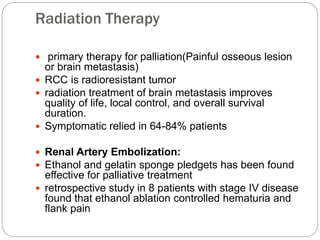
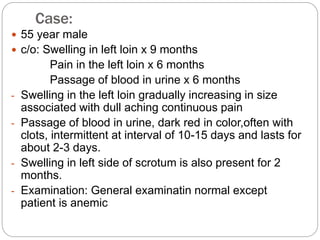
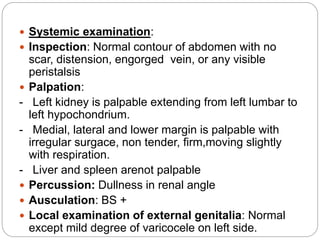
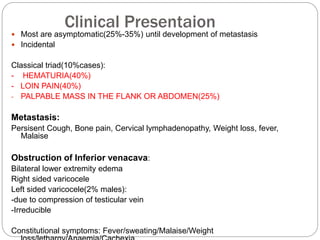
- Presentation may include hematuria, loin pain, and palpable abdominal mass. Metastasis can cause cough or bone pain.
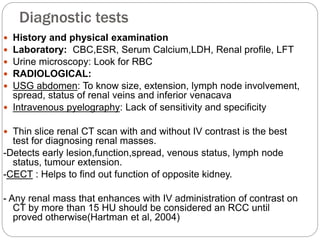
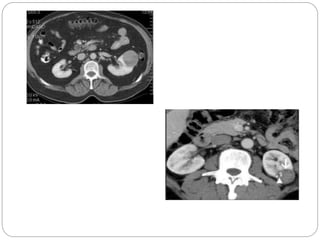
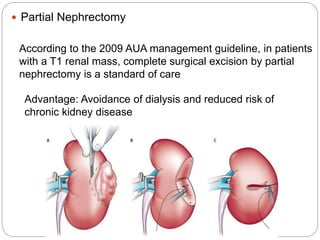
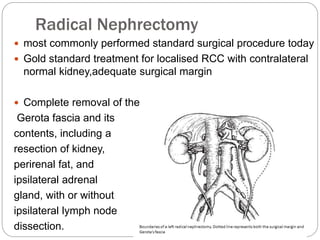
- Diagnosis involves imaging like CT scan and lab tests. Surgery is the main treatment but immunotherapy and targeted drugs are also used.
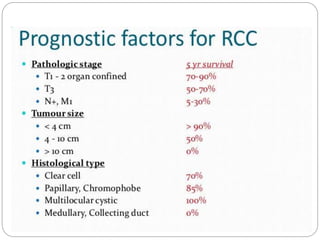
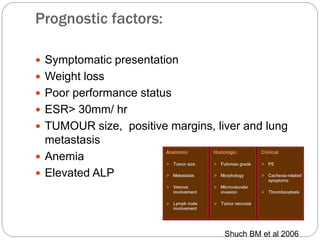
- Prognosis depends on stage - early stage has good prognosis but late stage with metastasis has poorer outlook.



![Risk Factors:
- Tobacco Smoking(24-30% cases)
2 fold increase risk for RCC
- Obesity , HTN
- Cystic Kidney disease patient after long term dialysis
- Hormonal : Diethylistillbestrol
- Occupational: Leather tanning, shoe workers,asbestos
Genetic:
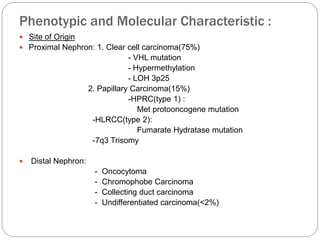
- Von Hippel-Lindau (VHL) syndrome [ 3p25] :
Bilateral
- Hereditary papillary renal carcinoma (HPRC)
- Familial renal oncocytoma (FRO) associated
with Birt-Hogg-Dube syndrome (BHDS)
- Hereditary renal carcinoma (HRC)
- Analgesic(Phenacetin)
- Family History Positive : 2.5 x greater risk
- Dietary: High fat/ Protein](https://image.slidesharecdn.com/renalcellcarcinoma-180406141019/85/Renal-cell-carcinoma-9-320.jpg)







![Chemotherapy
Floxuridine (5-fluoro 2'-deoxyuridine [FUDR])
5-fluorouracil (5-FU)
Vinblastine
paclitaxel (Taxol)
carboplatin
ifosfamide
gemcitabine
anthracycline (doxorubicin)
RCC is refractory to most chemotherapeutic agents
because of multidrug resistance mediated by p-
glycoprotein](https://image.slidesharecdn.com/renalcellcarcinoma-180406141019/85/Renal-cell-carcinoma-35-320.jpg)