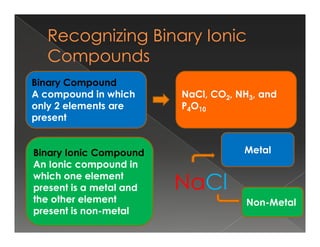
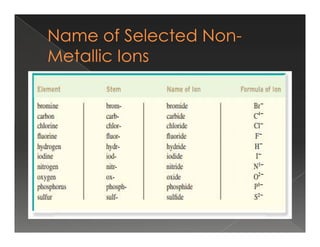
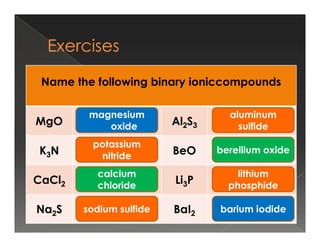
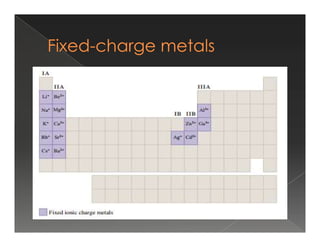
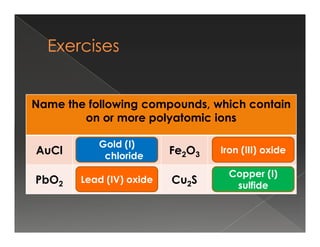
This document discusses binary compounds and ionic bonding. It defines binary compounds as those containing only two elements and provides examples such as NaCl, CO2, NH3, and P4O10. It specifies that a binary ionic compound contains a metal and non-metal. Rules for naming ionic compounds are outlined, including noting the charge of the metal ion. Methods for determining the charge on the metal ion by balancing charges are also described.