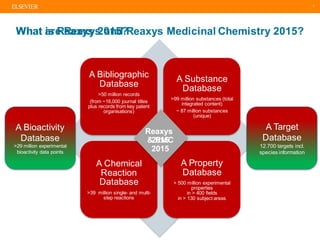
This document discusses structure searching in Reaxys, beginning with an introduction to Reaxys and its contents. It then covers essentials of structure searching such as supported structure editors, differences between editor capabilities and Reaxys search features, and Reaxys' substance model. Examples of simple and sophisticated structure searching techniques are provided. The document concludes with an example of reaction similarity searching to find reactions related to a Diels-Alder reaction.