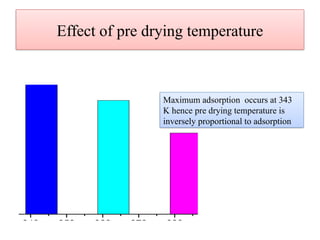
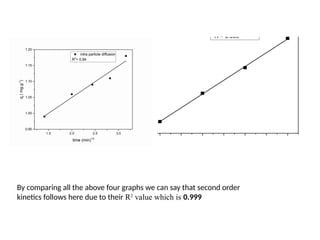
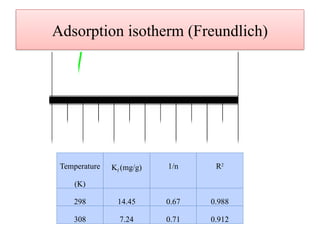
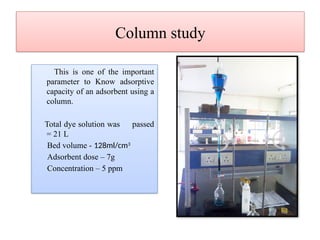
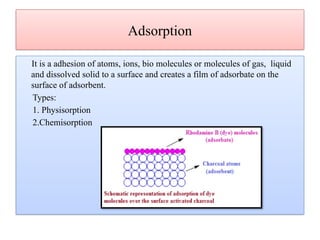
This document discusses the effective biosorption of malachite green dye from aqueous solutions using saal flower as a low-cost biosorbent, highlighting the adsorption mechanisms, conditions, and kinetic studies. It presents experimental results showing the maximum adsorption capacity, the effects of various parameters, and concludes that the adsorption process follows second-order kinetics and Langmuir isotherm. The study also acknowledges contributions from various individuals and institutions in conducting the research.



![About Malachite green
• λmax= 617 nm
• Cationic dye
• pH sensitive (green colour arises between
pH (3-11)
• Green crystal
• Soluble in ethanol, methanol, amyl alcohol
and water
• Highly toxic to mammalian cells and liver
tumor enhancing agent
• Produces hazardous product when heated
to decomposition
• [4-[[4-(dimethylamino)phenyl]-
phenylmethylidene]cyclohexa-2,5-dien-1-
ylidene]-dimethylazanium;2-hydroxy-2-
oxoacetate;oxalic acid
• Chemical formula : C52H54N4O12 (dimer)](https://image.slidesharecdn.com/presentation1-241223062832-da3c6c57/85/Presentation-1-pptx-Msc-Research-Paper-8-320.jpg)