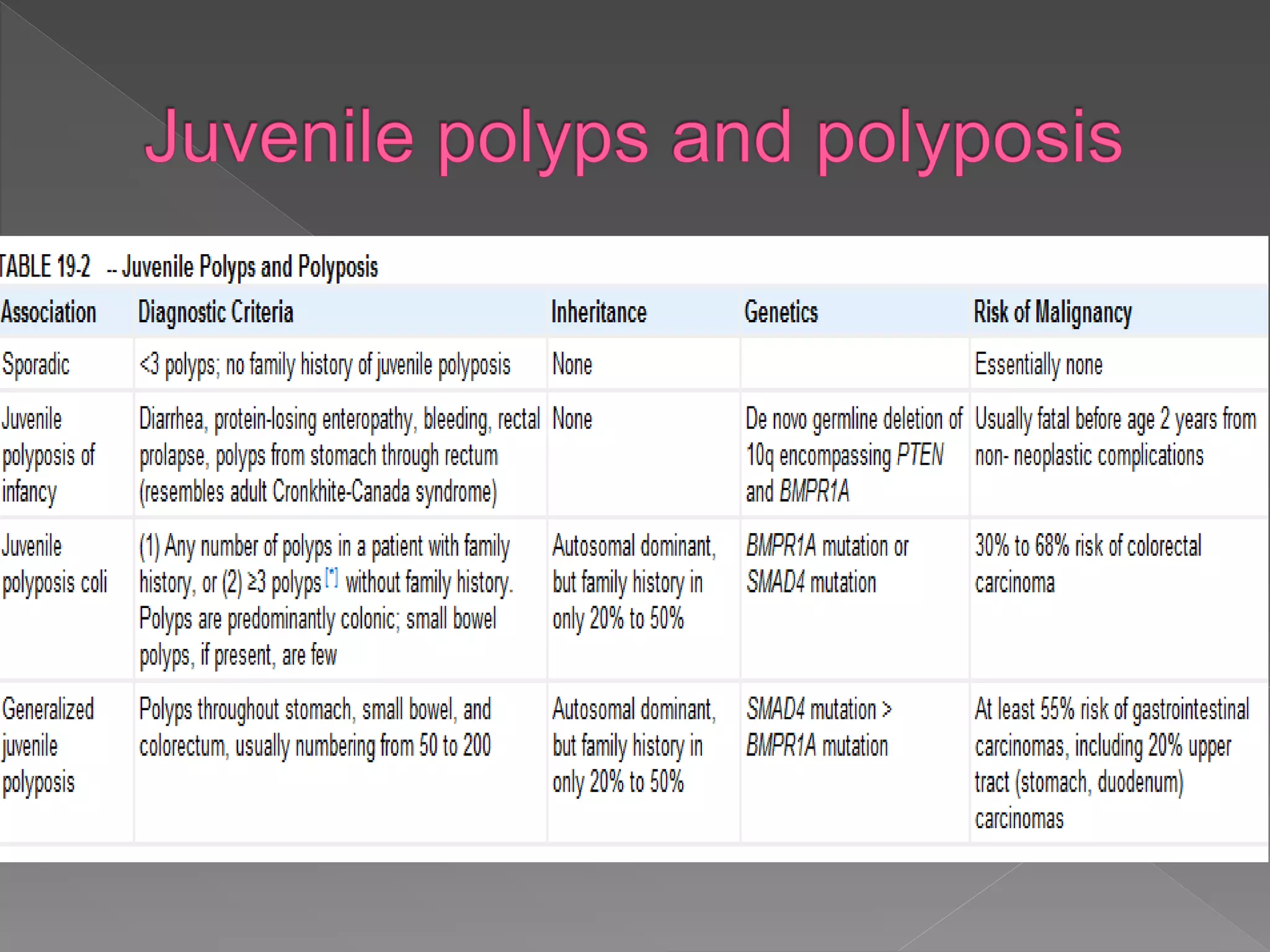
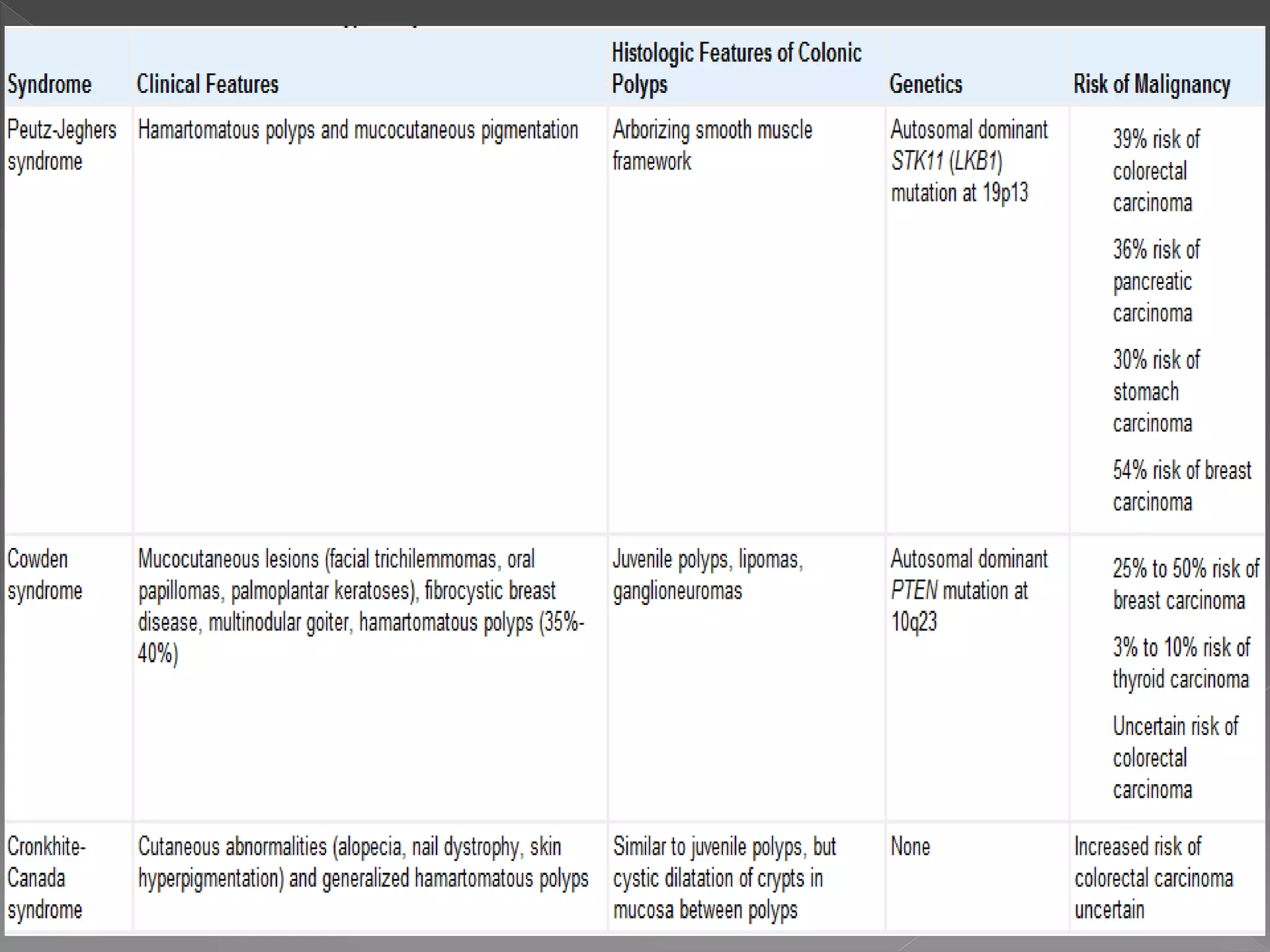
Colorectal carcinoma (CRC) is a prevalent cancer that often arises from benign polyps, making screening crucial for prevention. Key precursors include adenomatous and serrated polyps, which exhibit specific morphologic and molecular characteristics, influencing their progression to malignancy. Various polyp types, growth patterns, and genetic mutations play significant roles in the classification and treatment decisions for patients at risk.