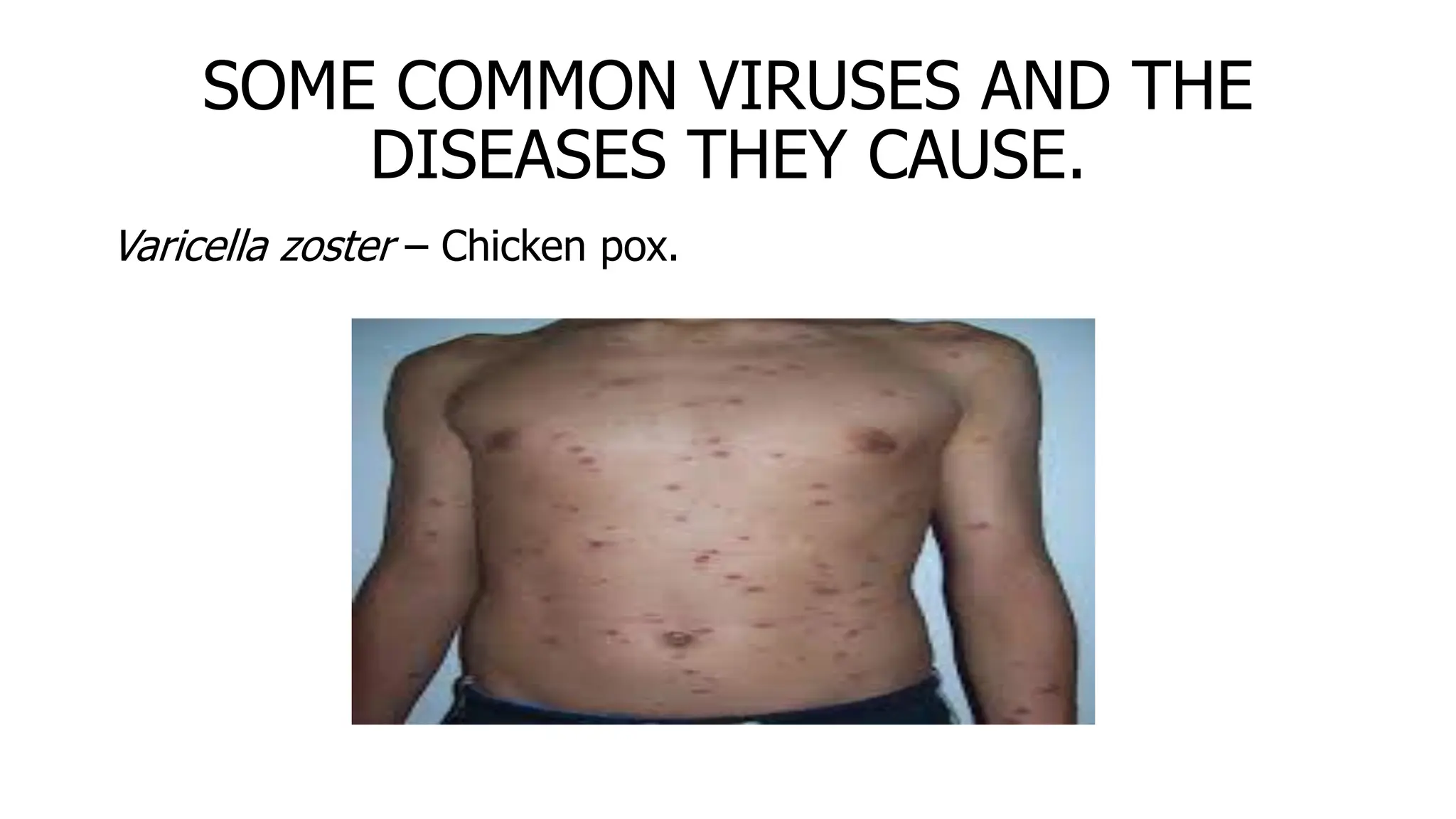
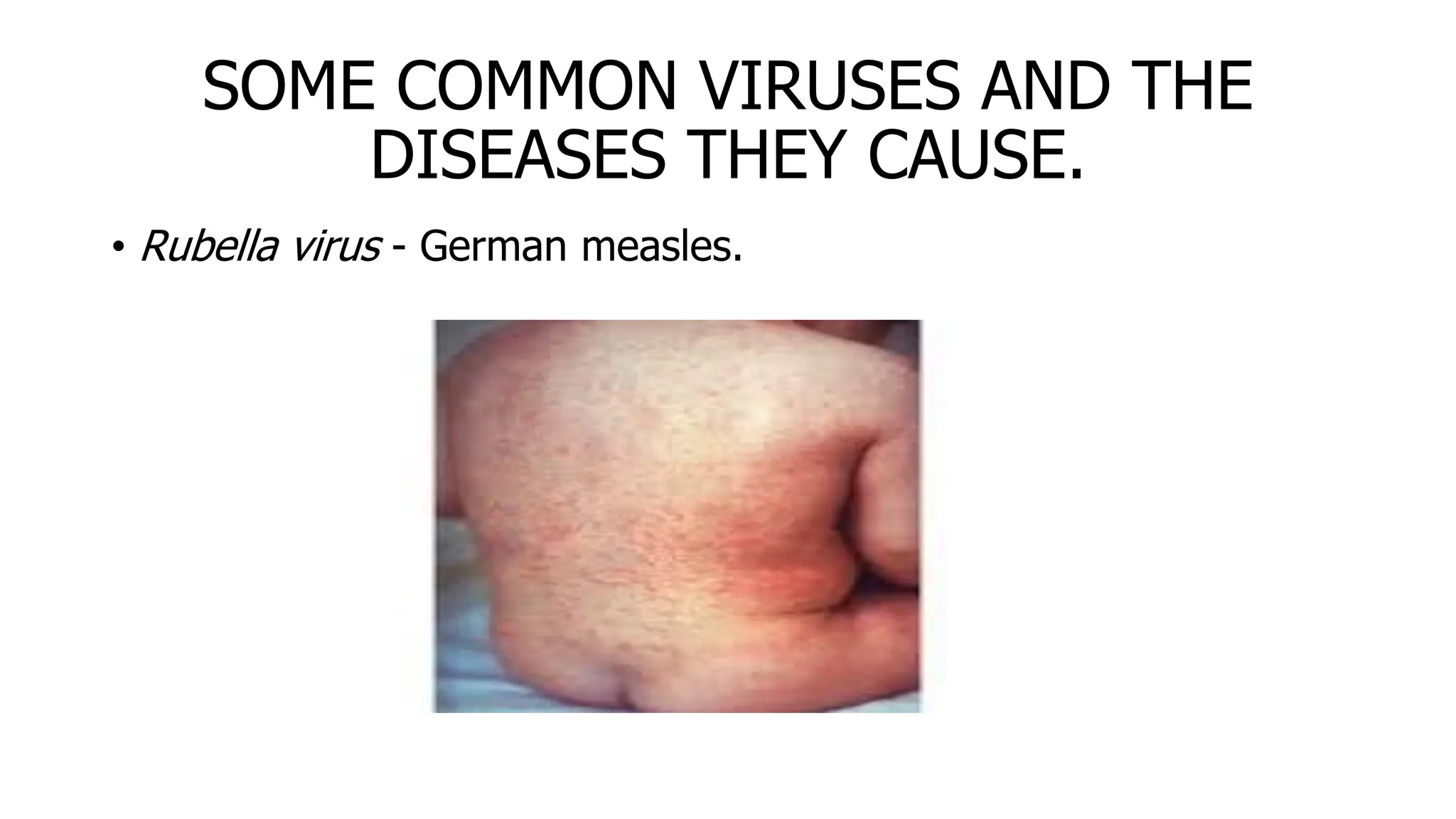
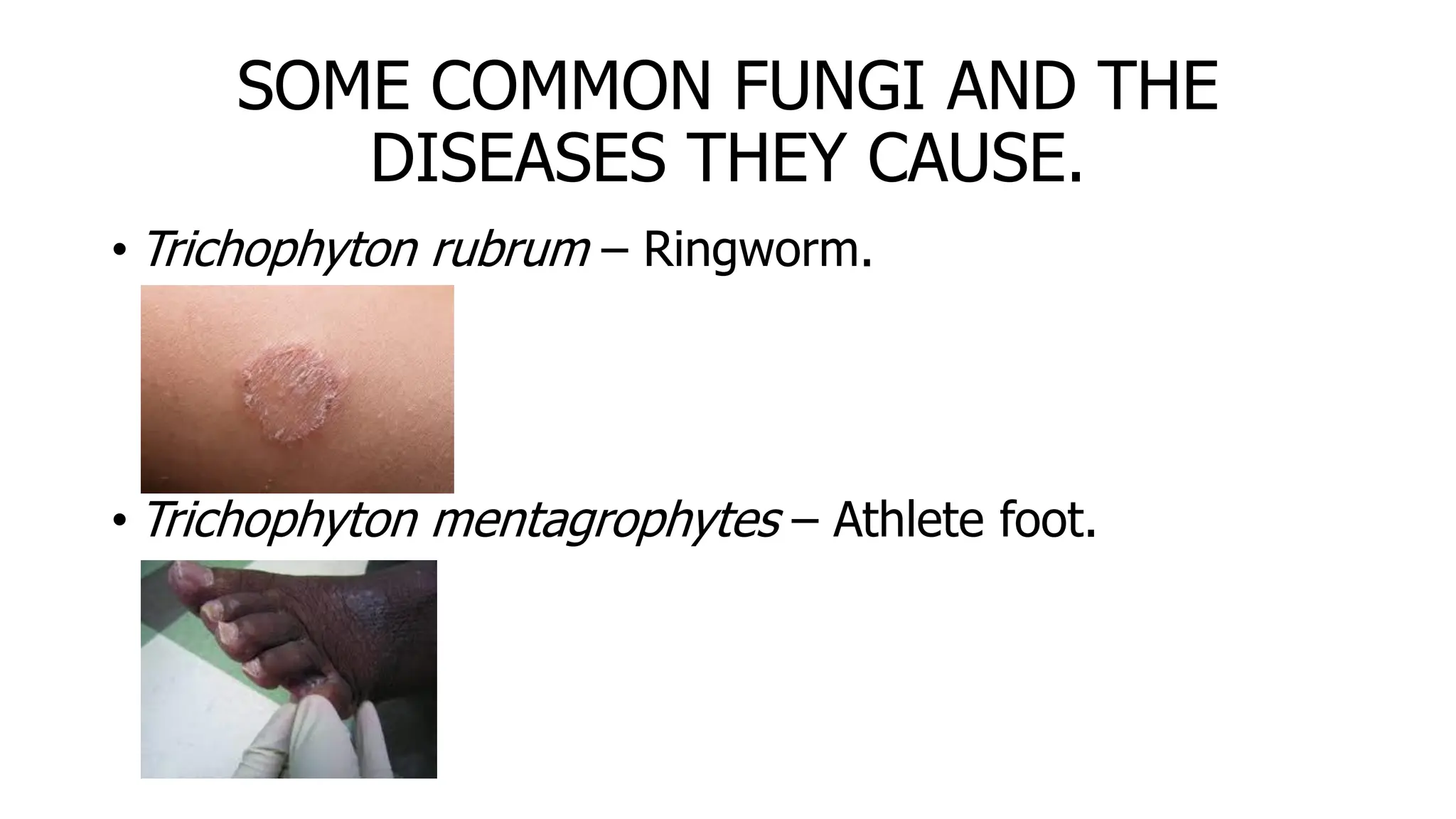
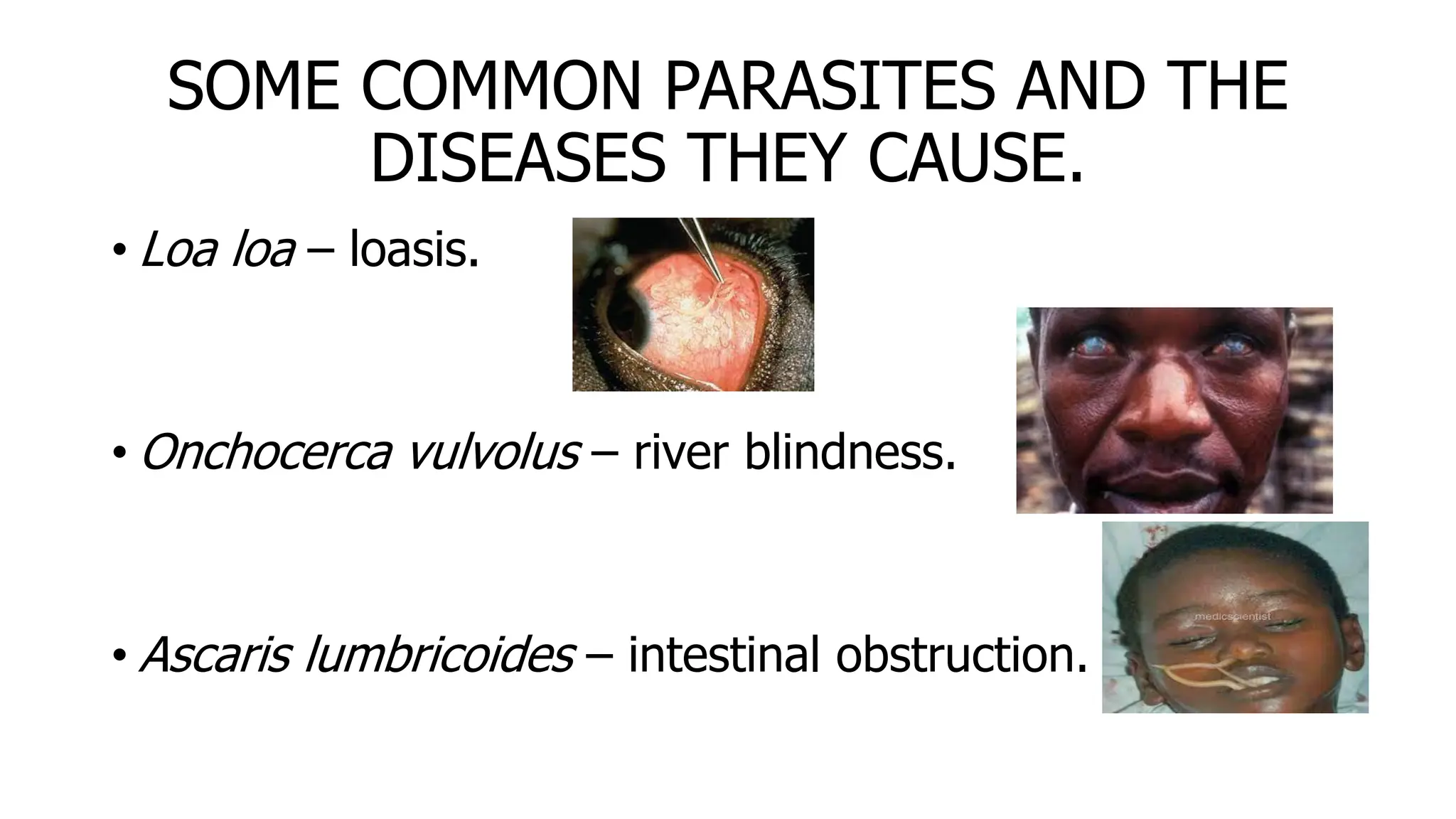
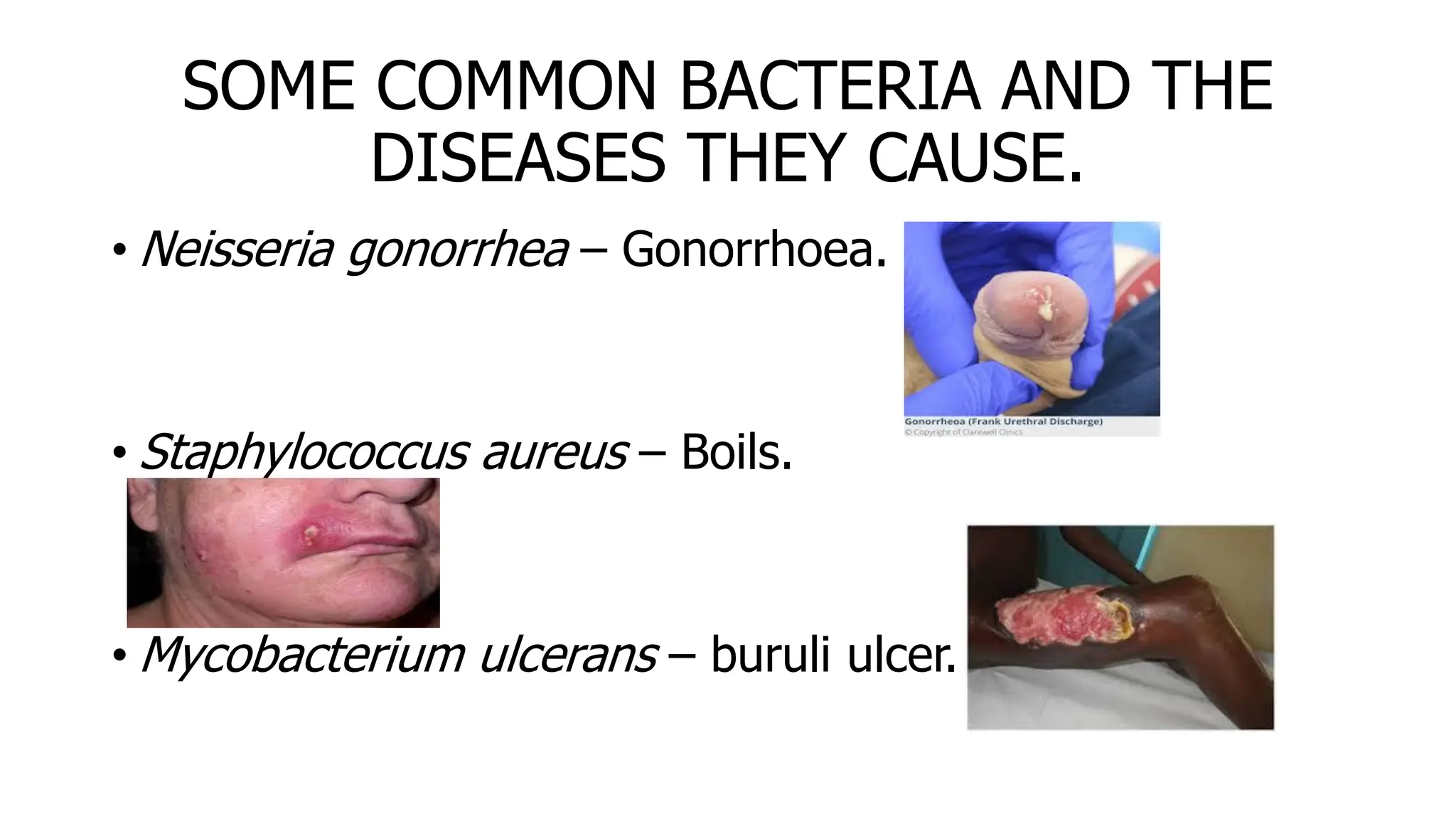
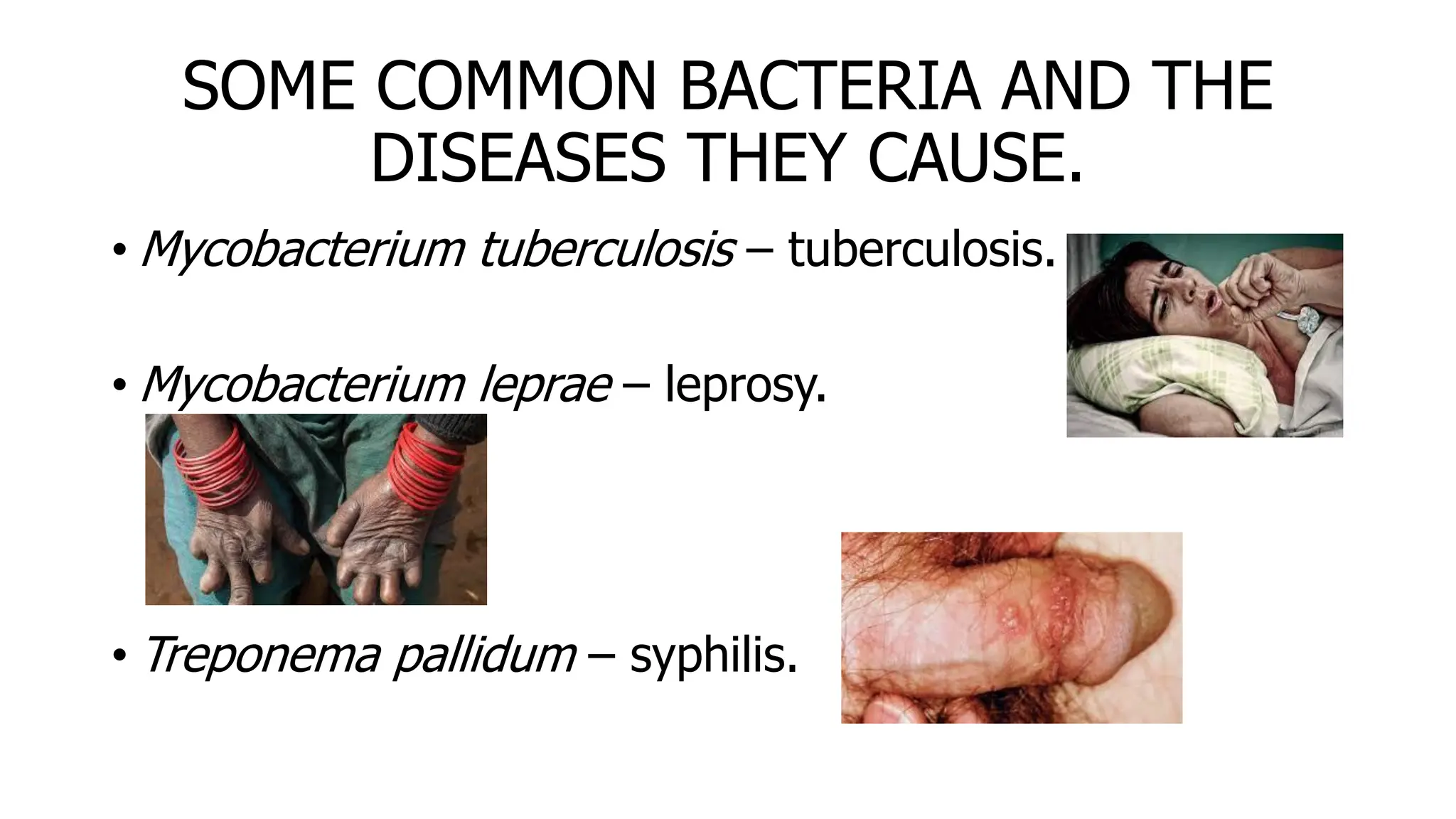
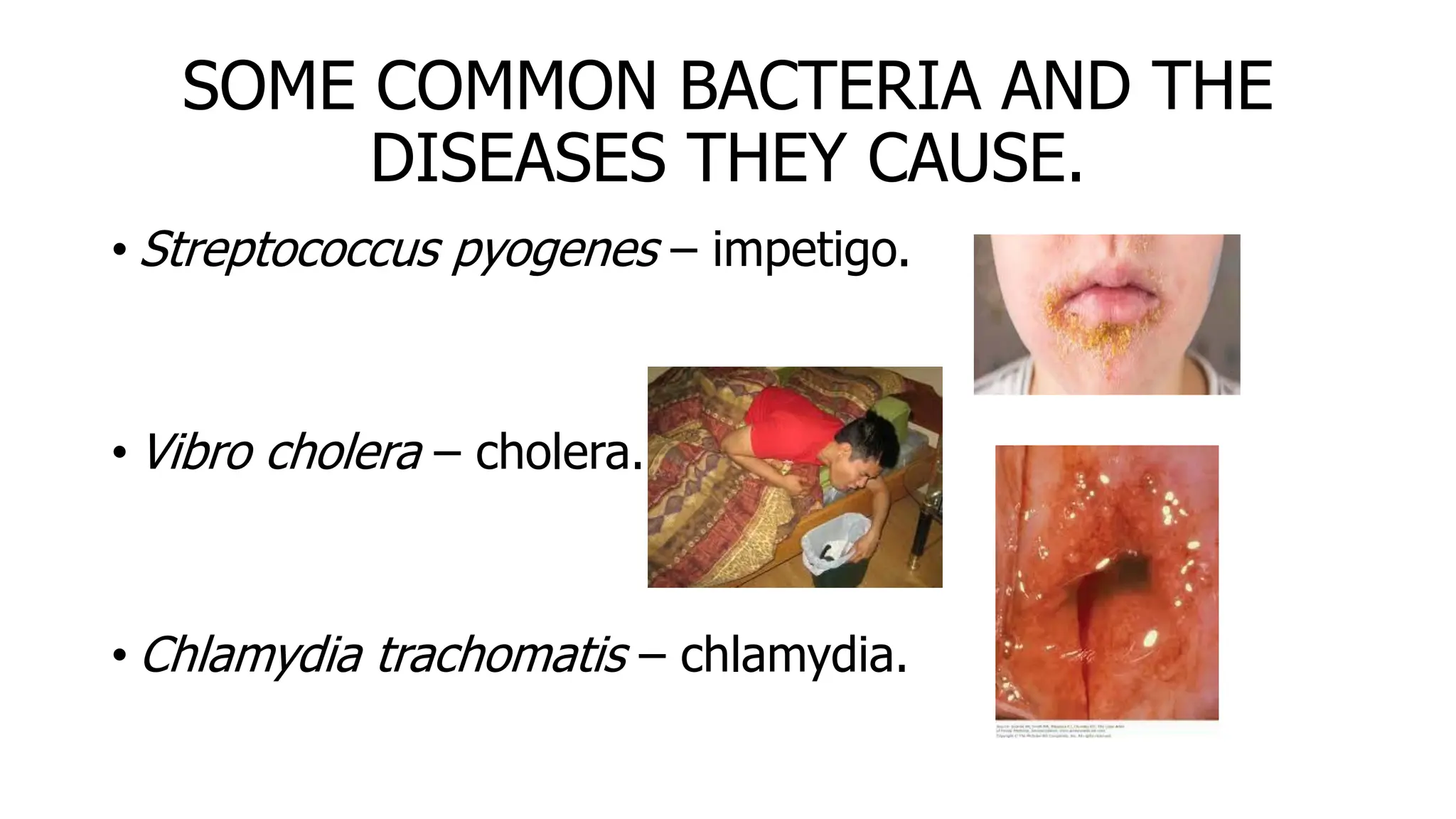
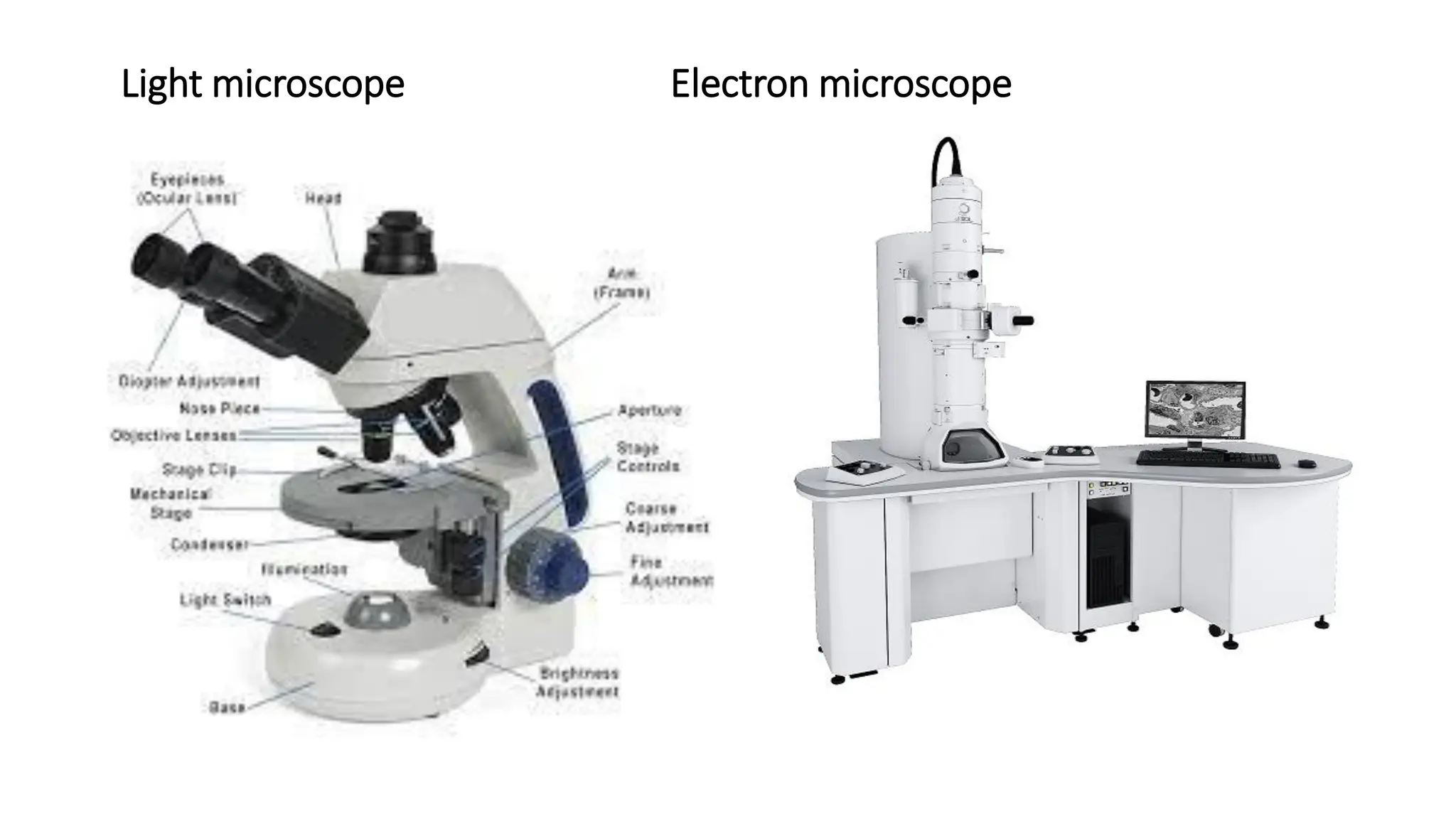
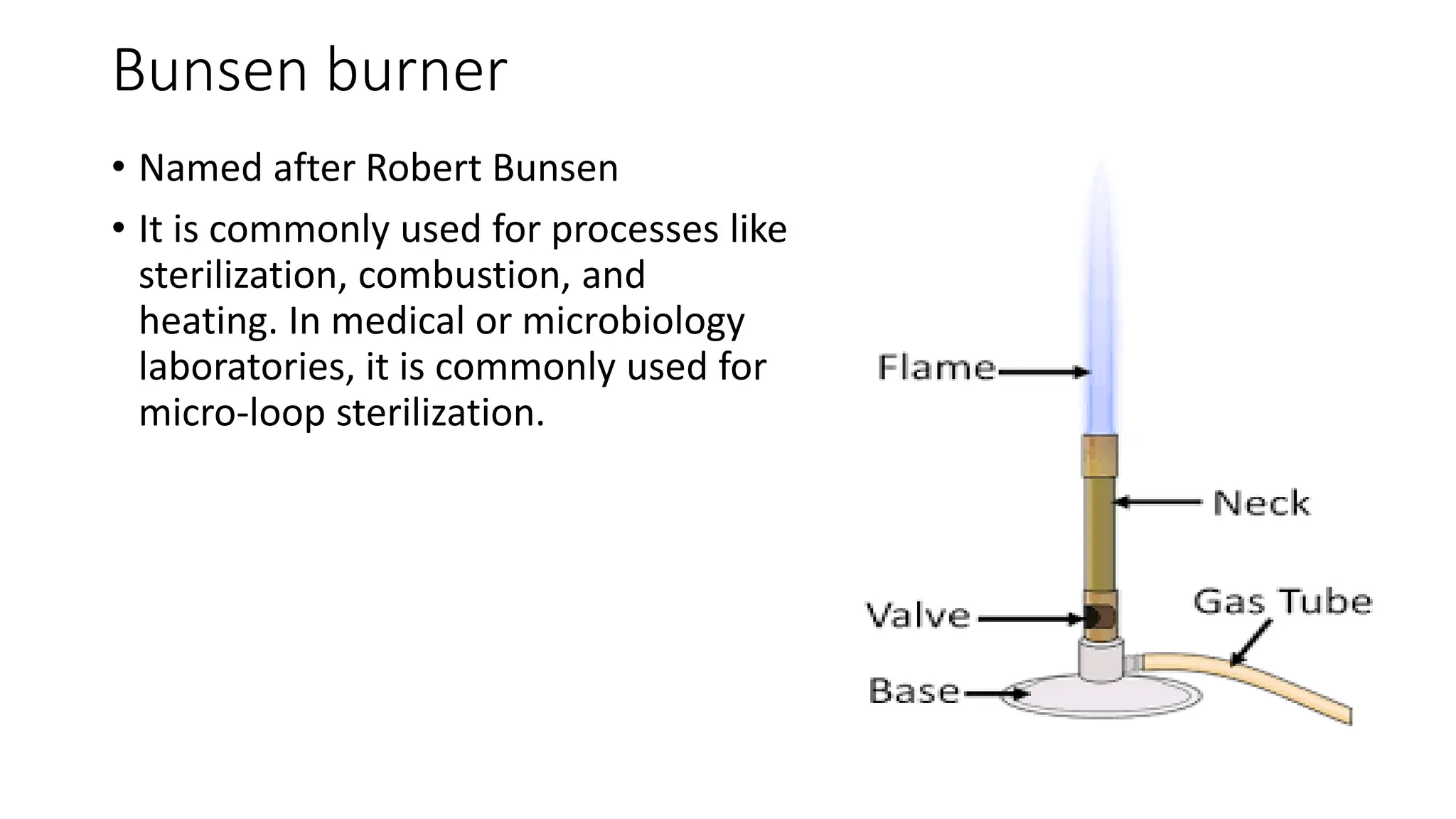
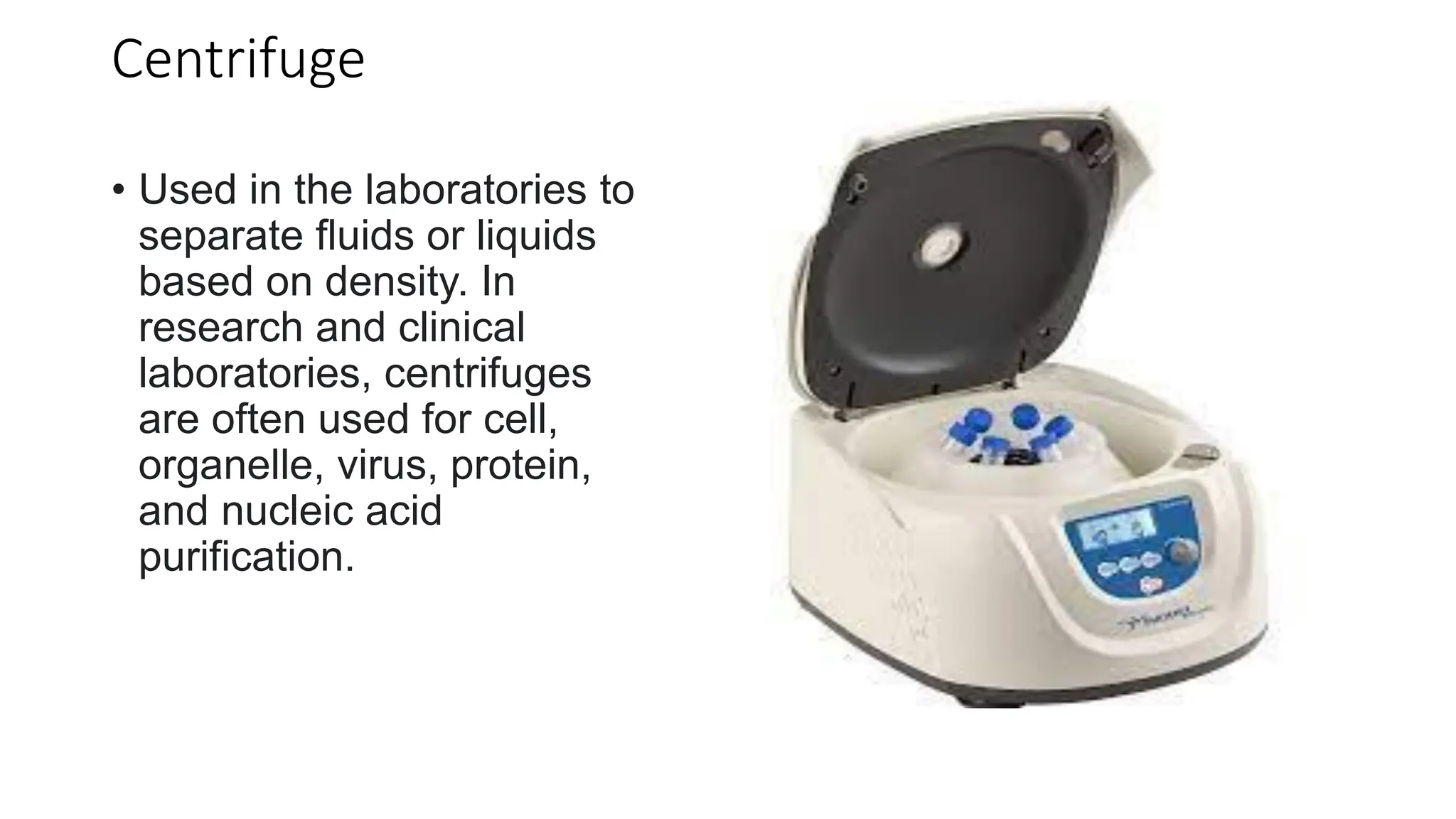
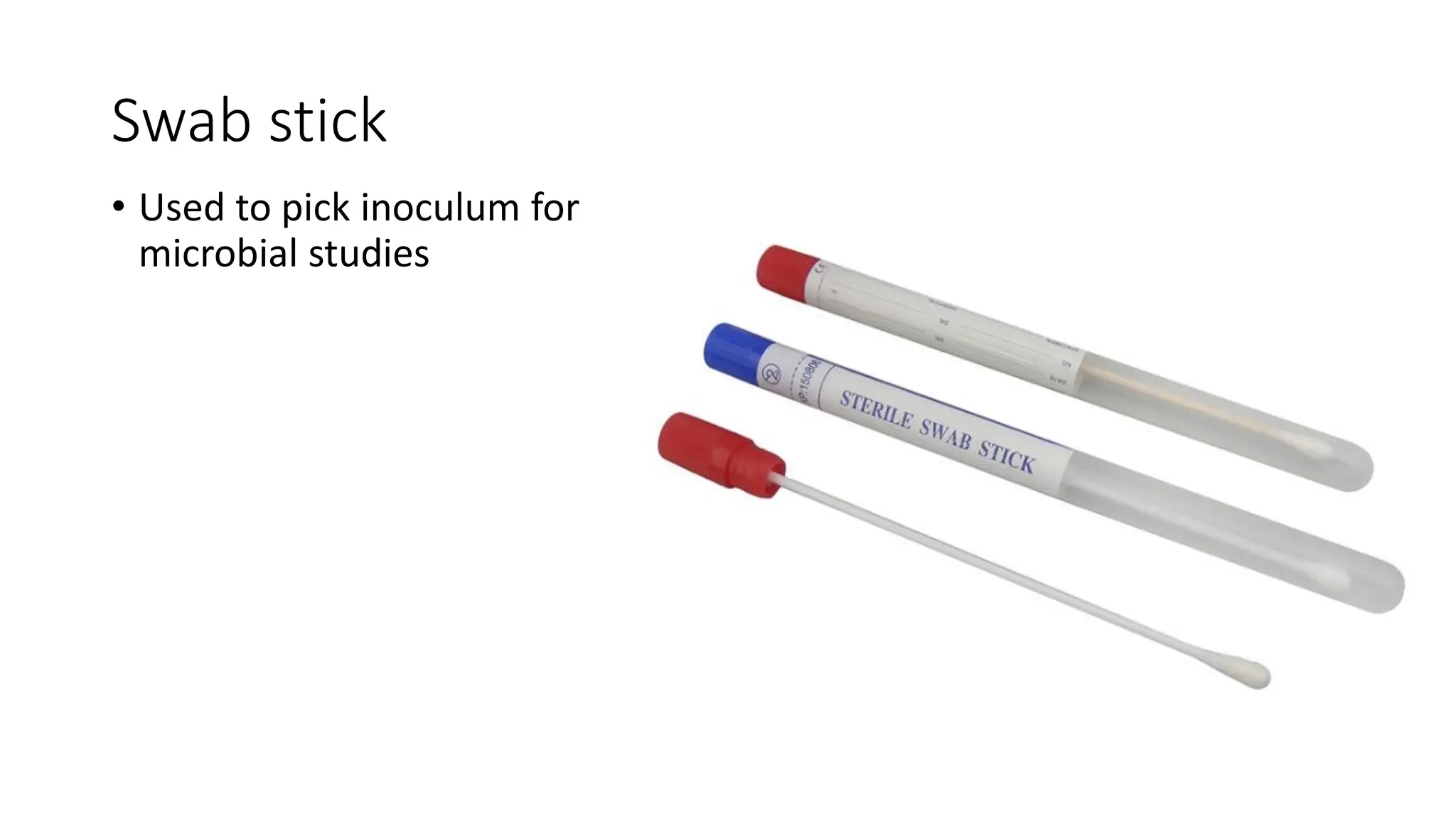
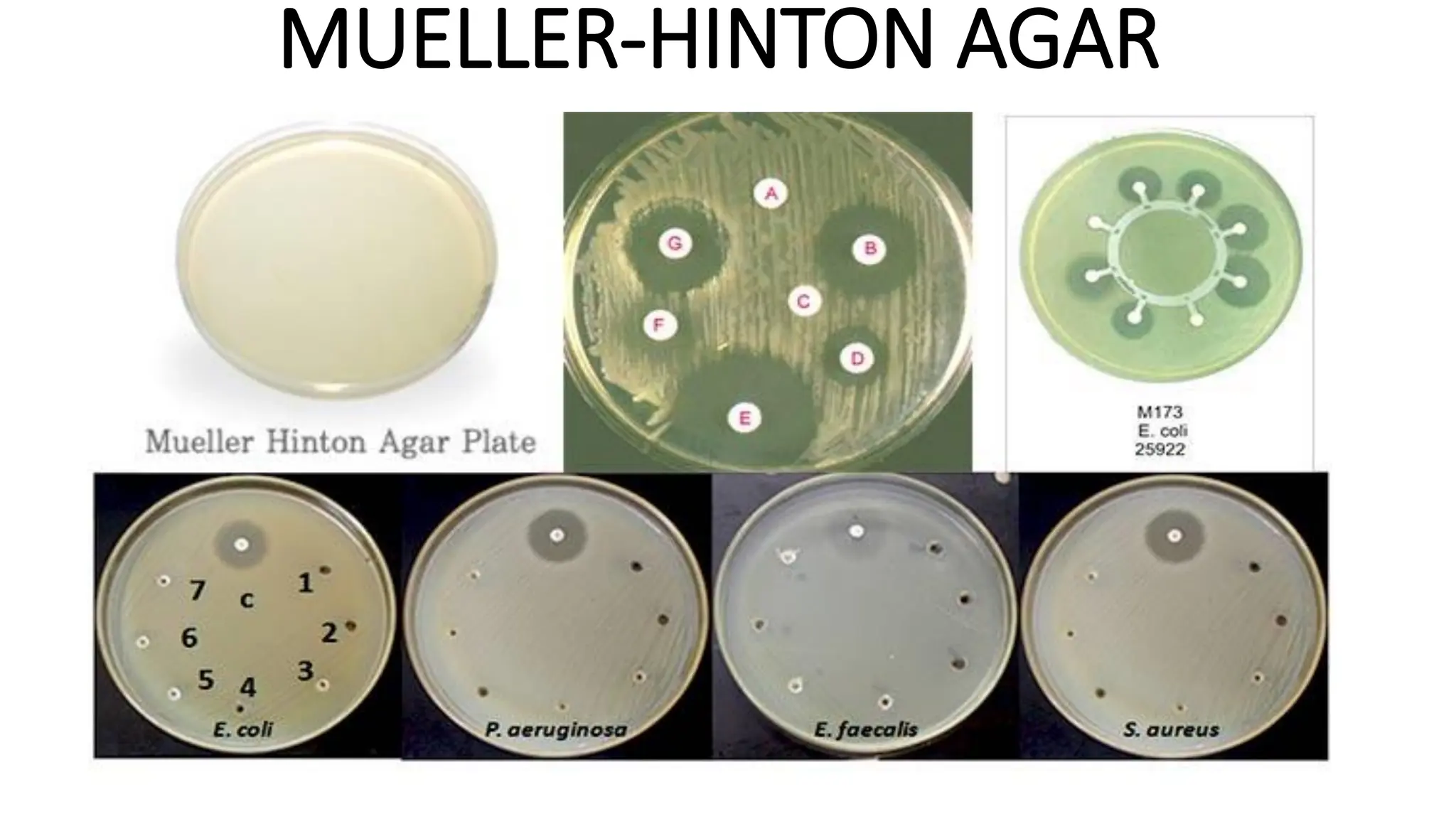
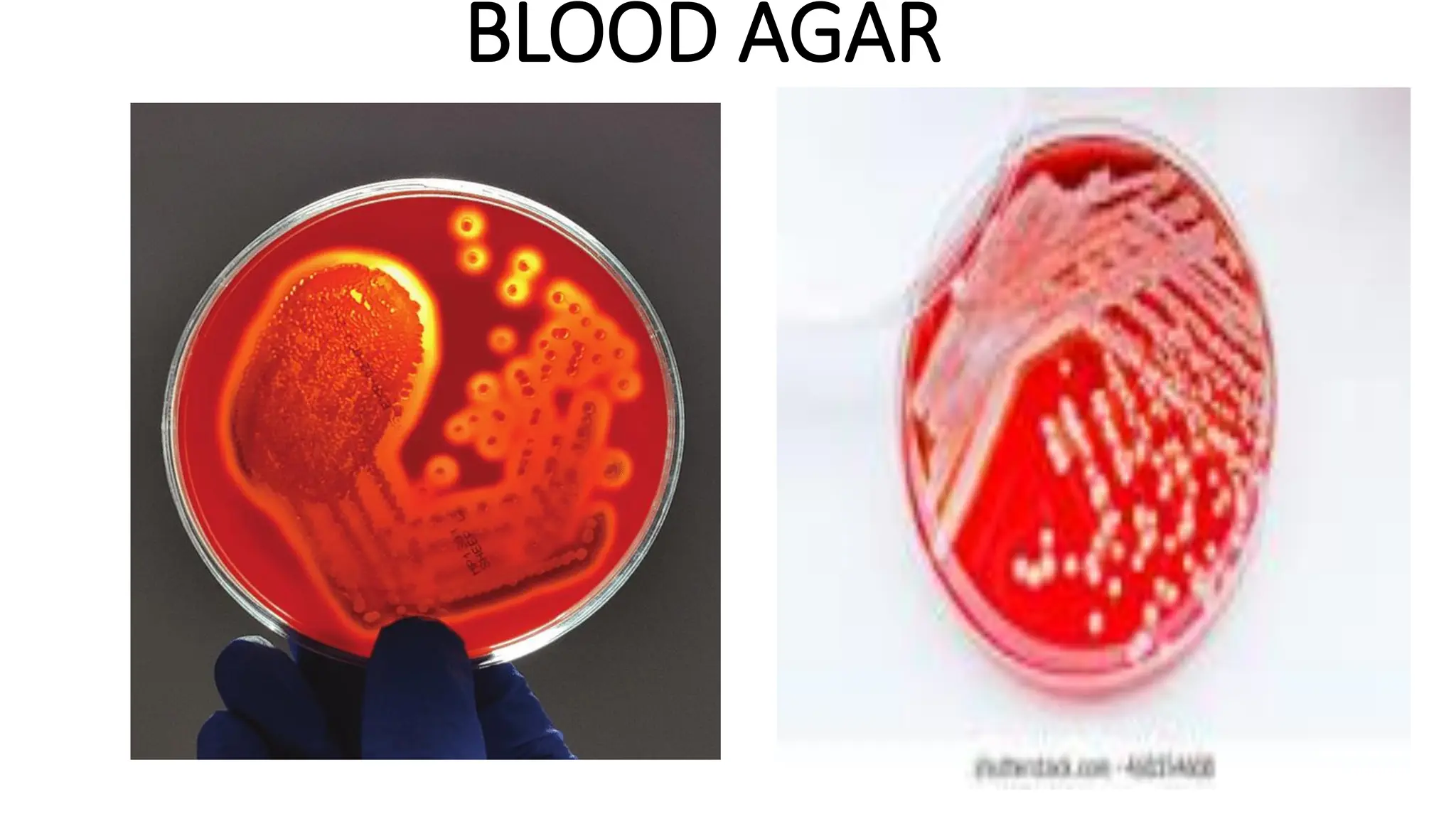
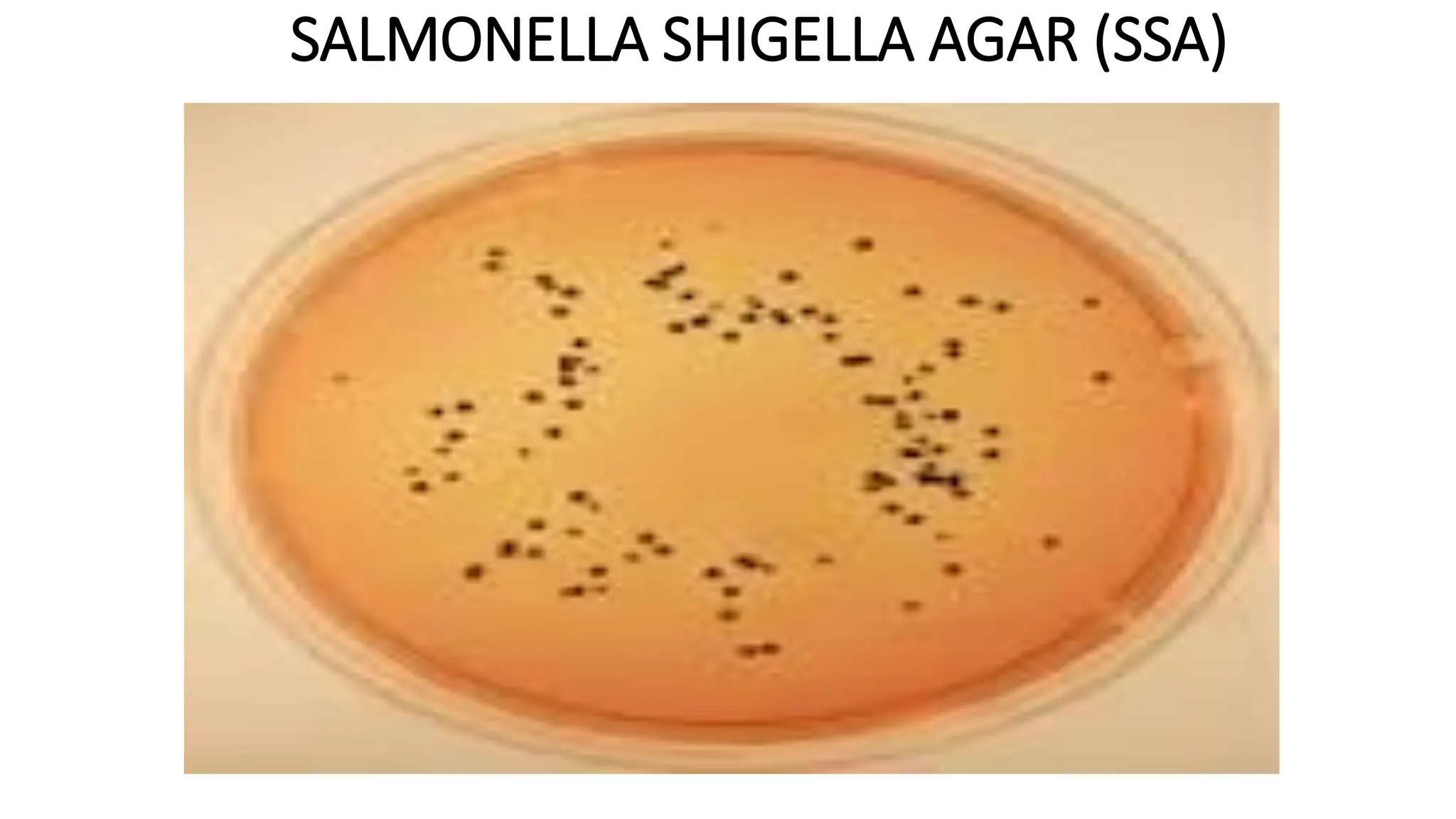
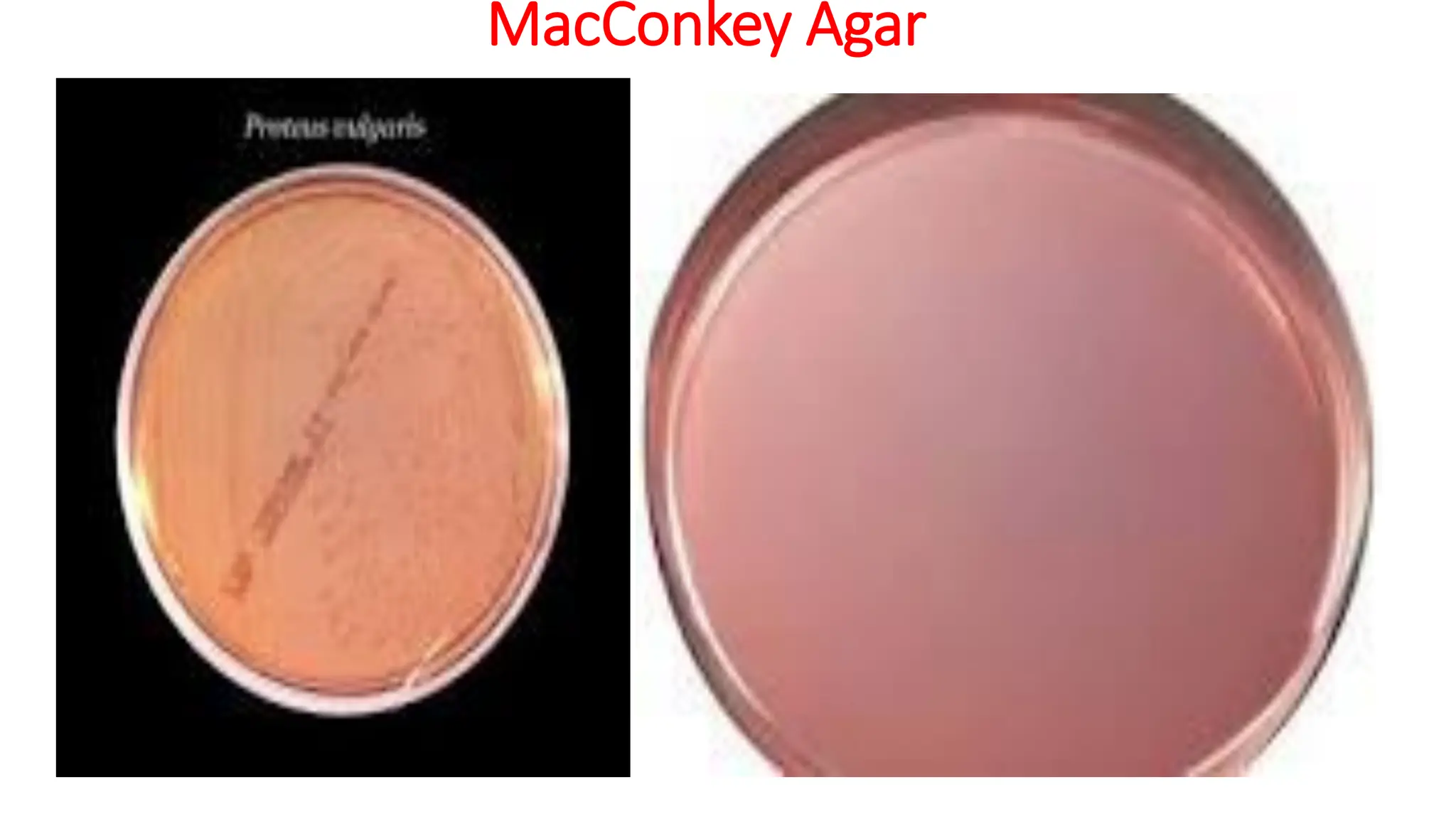
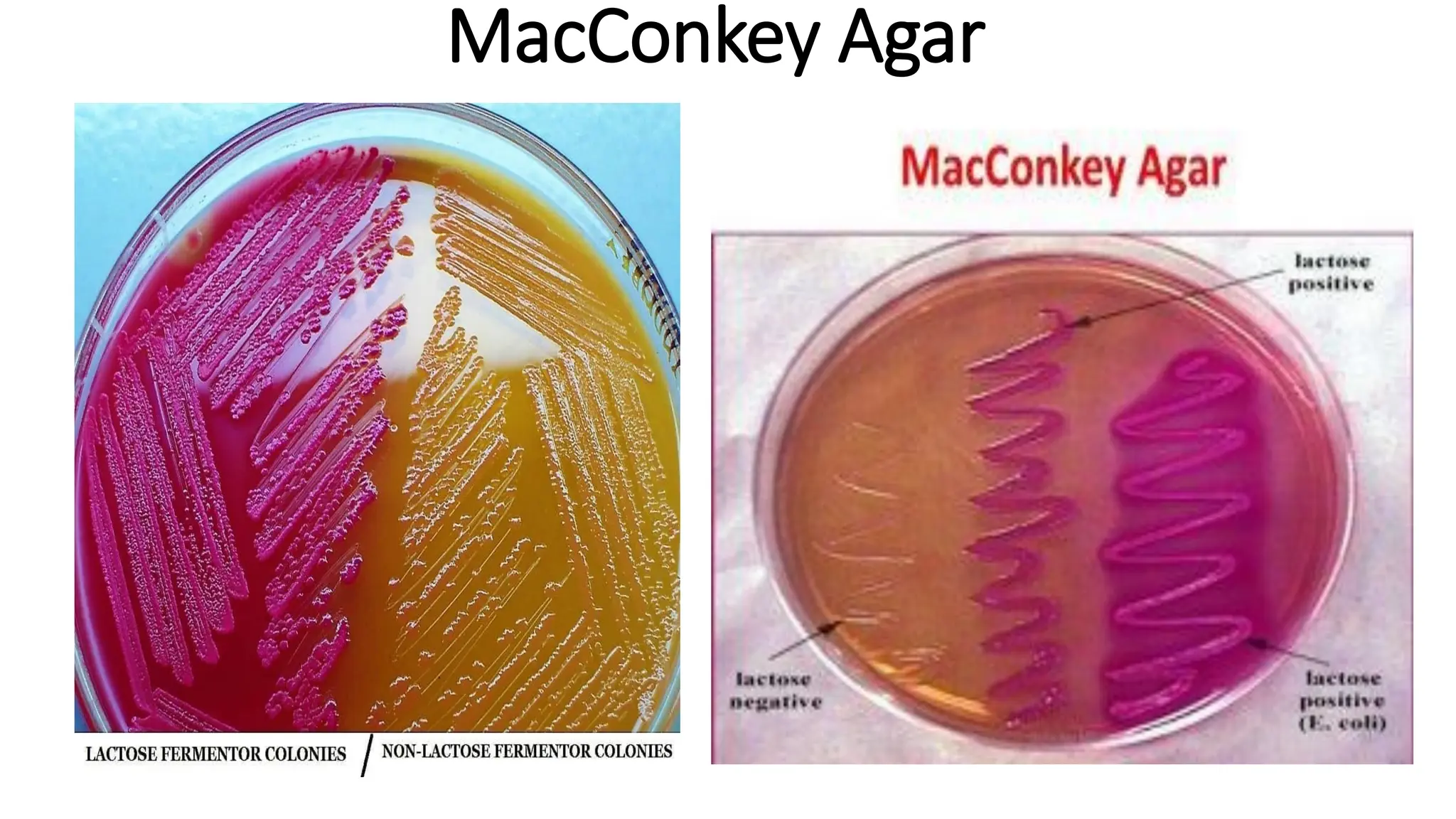
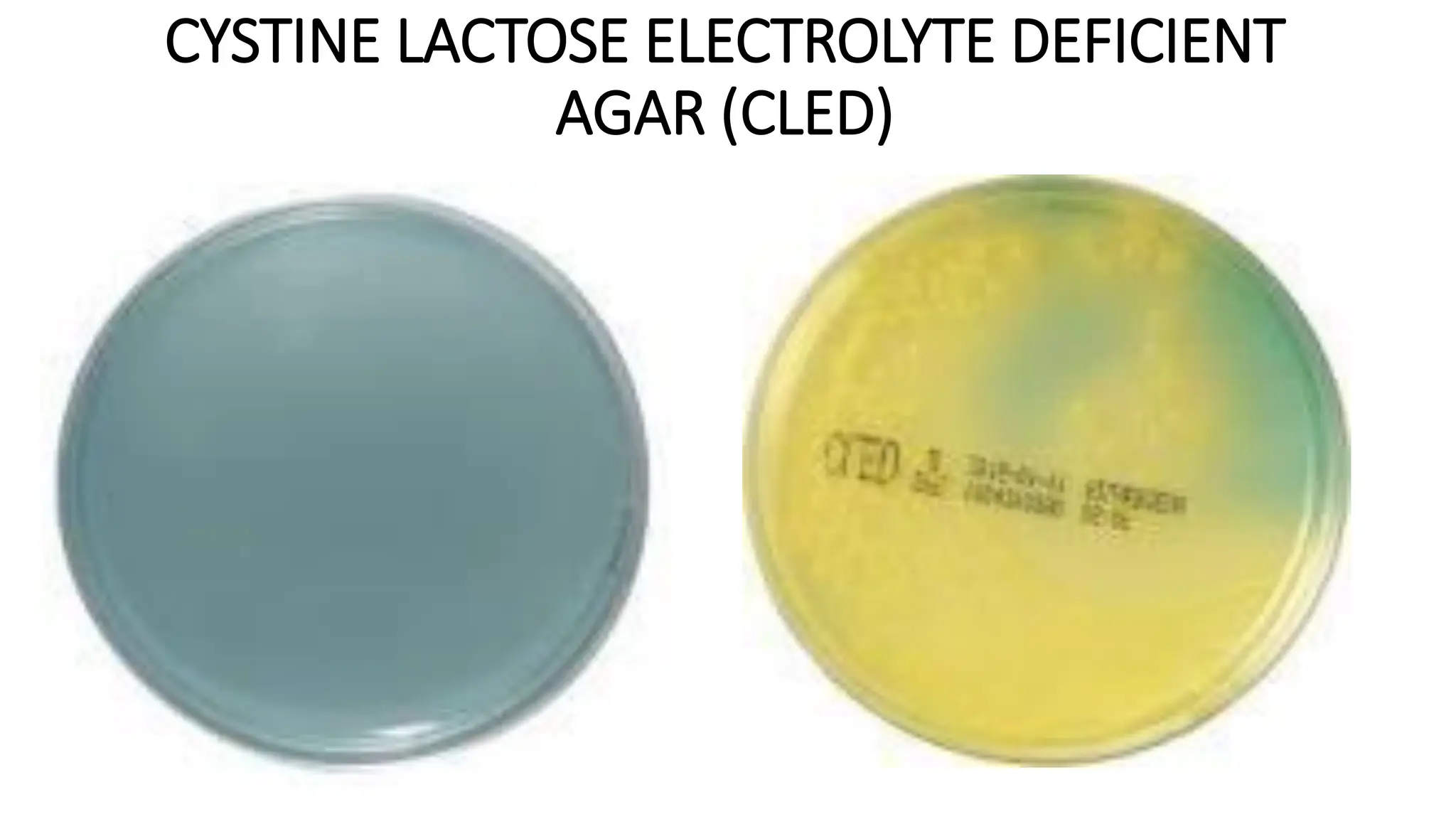
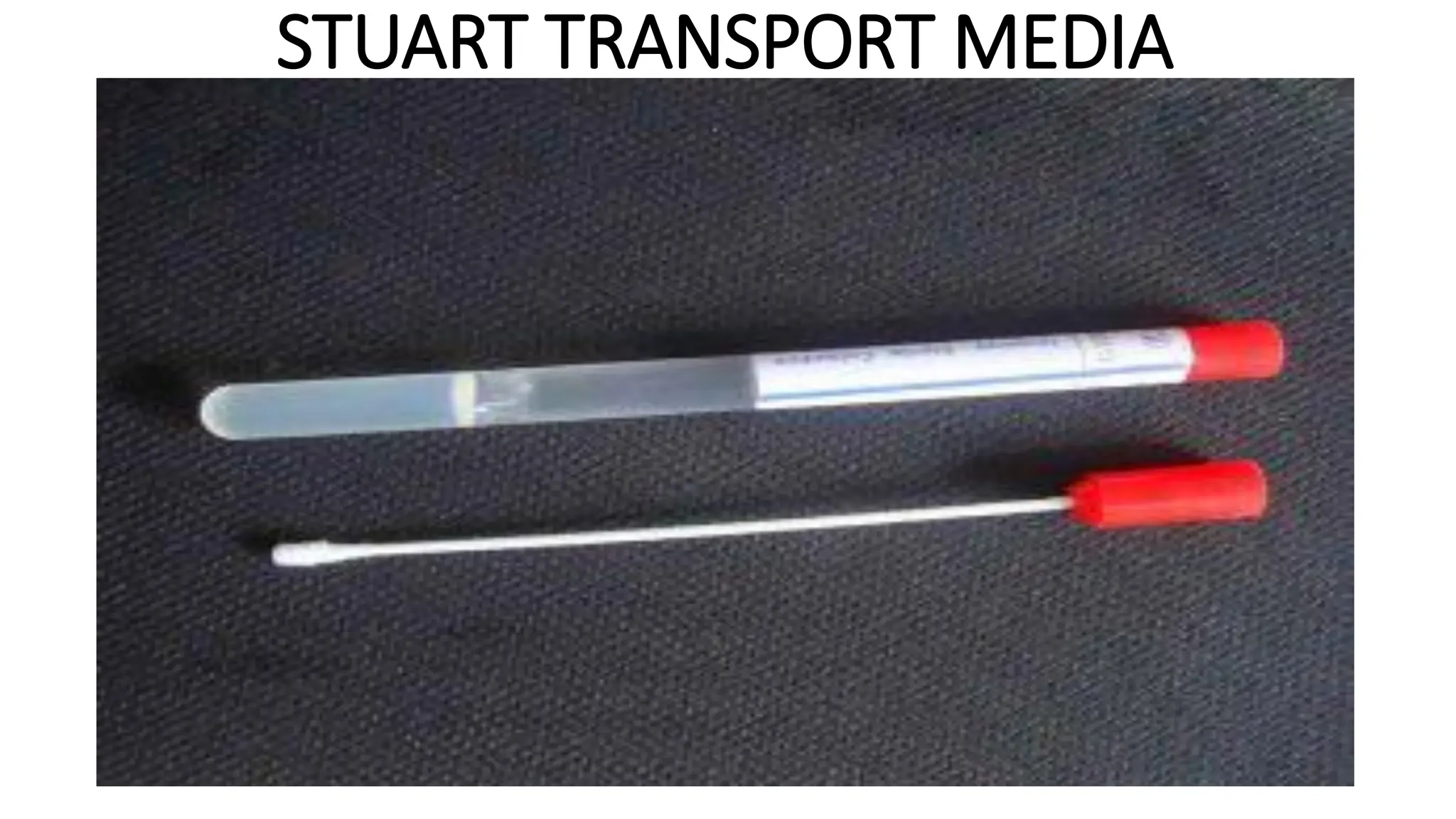
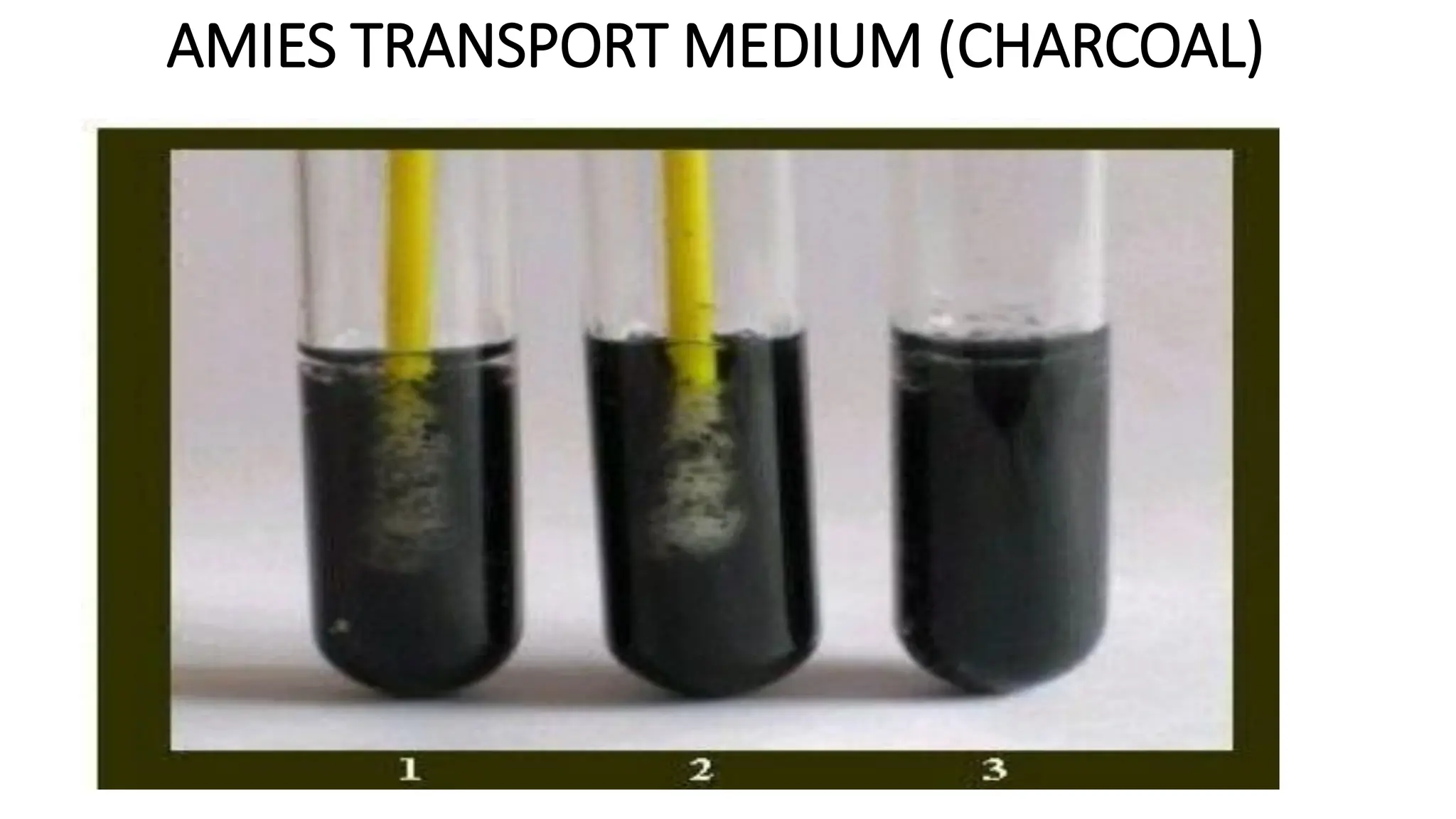
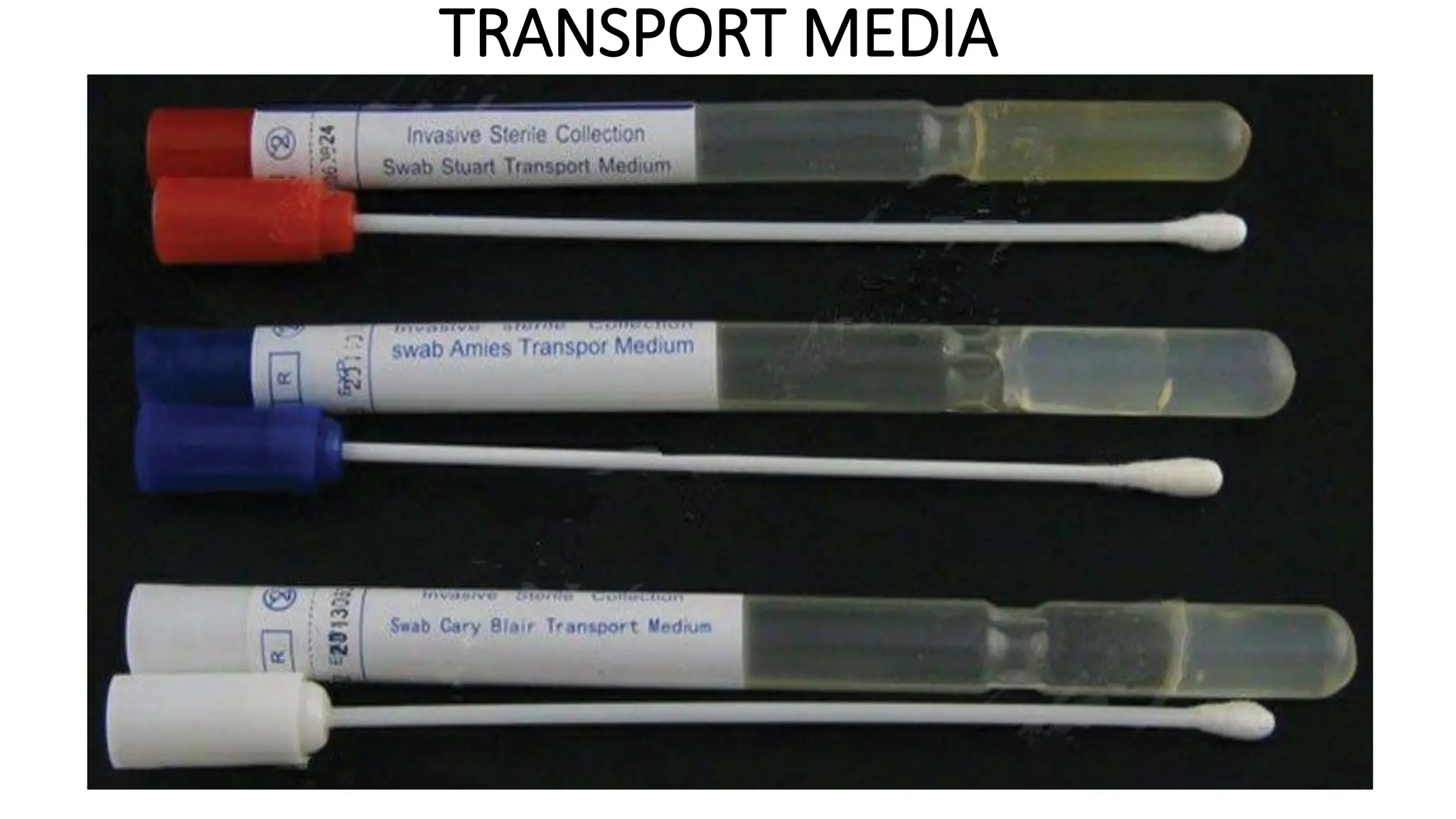
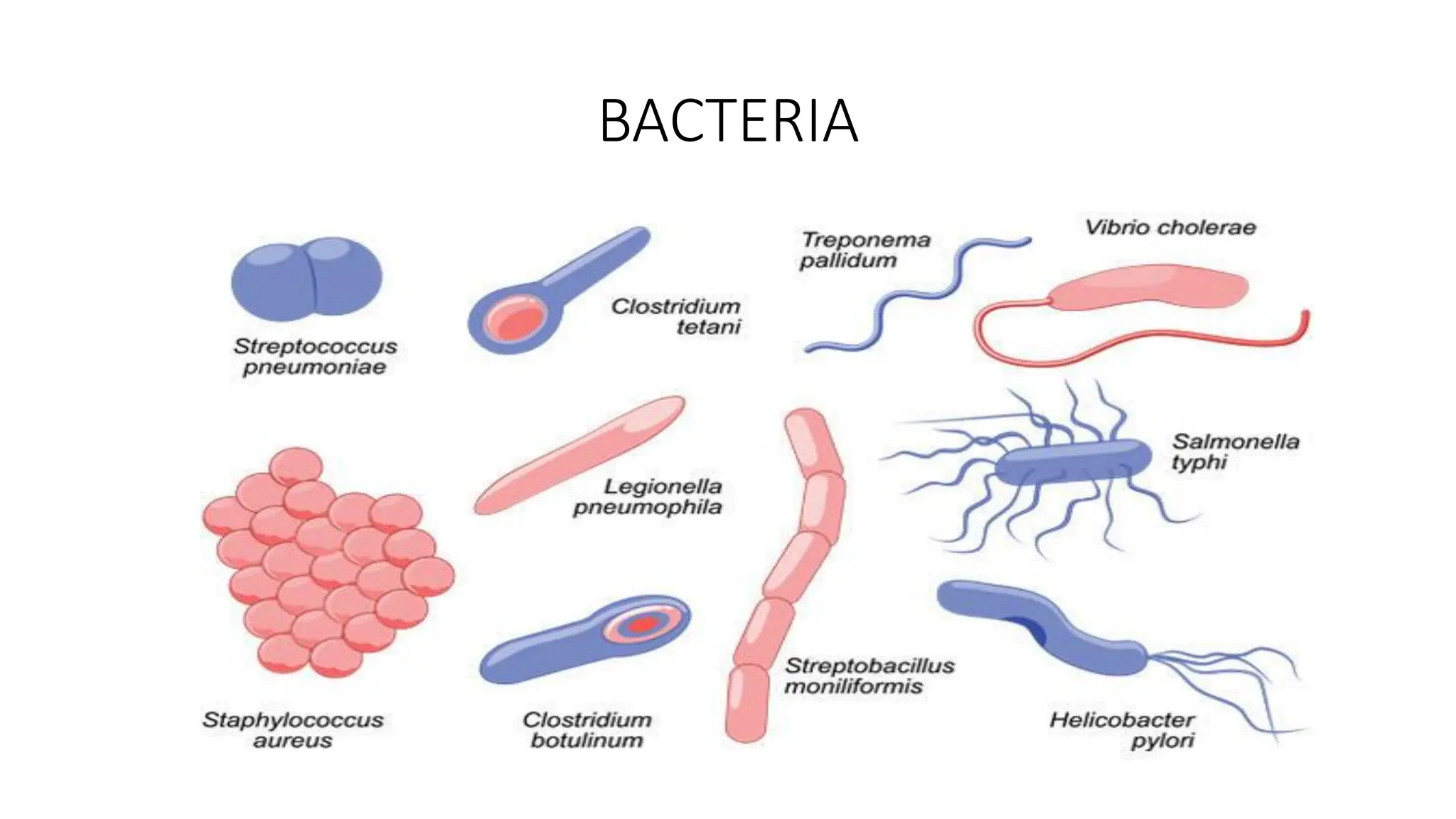
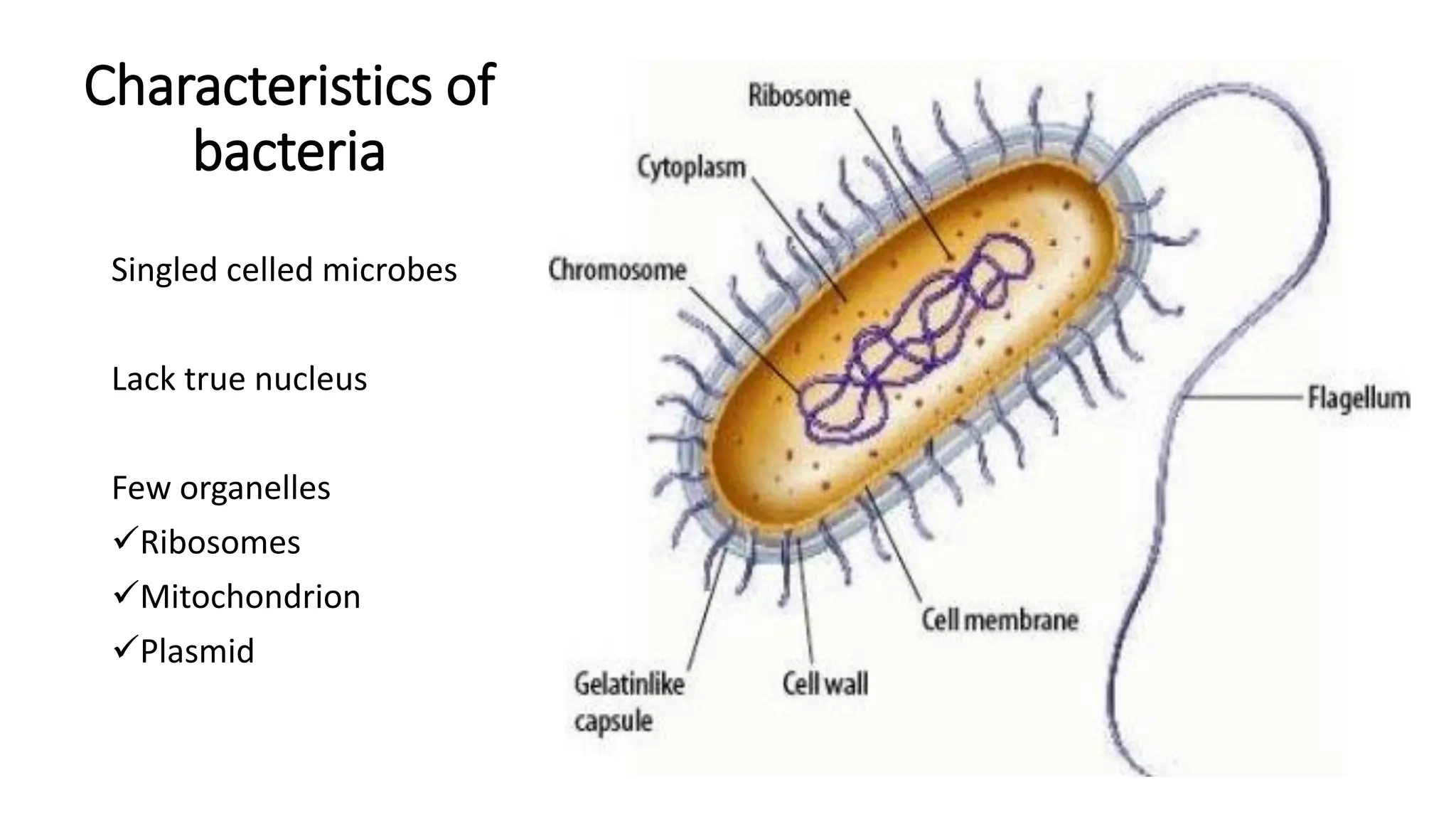
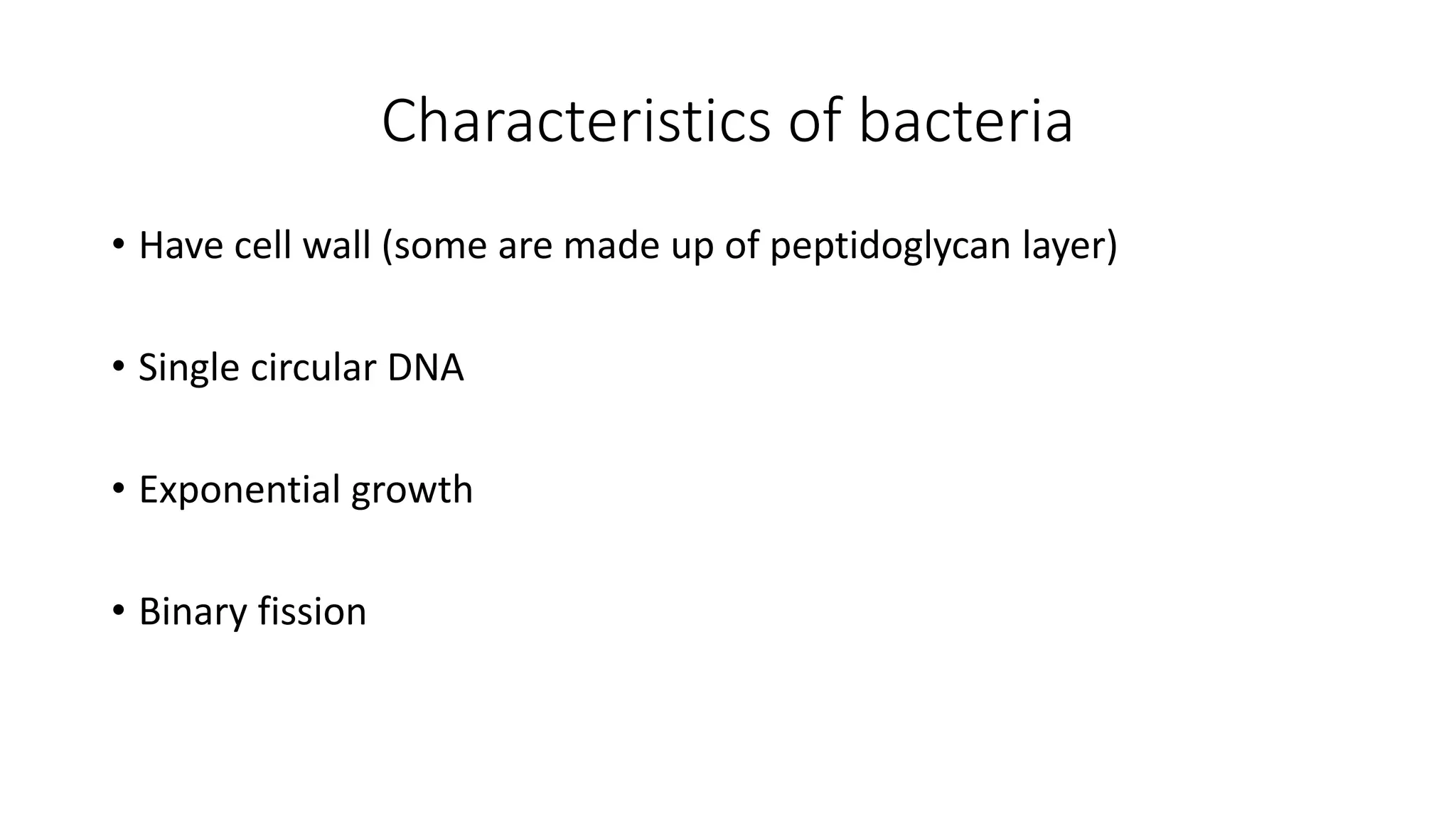
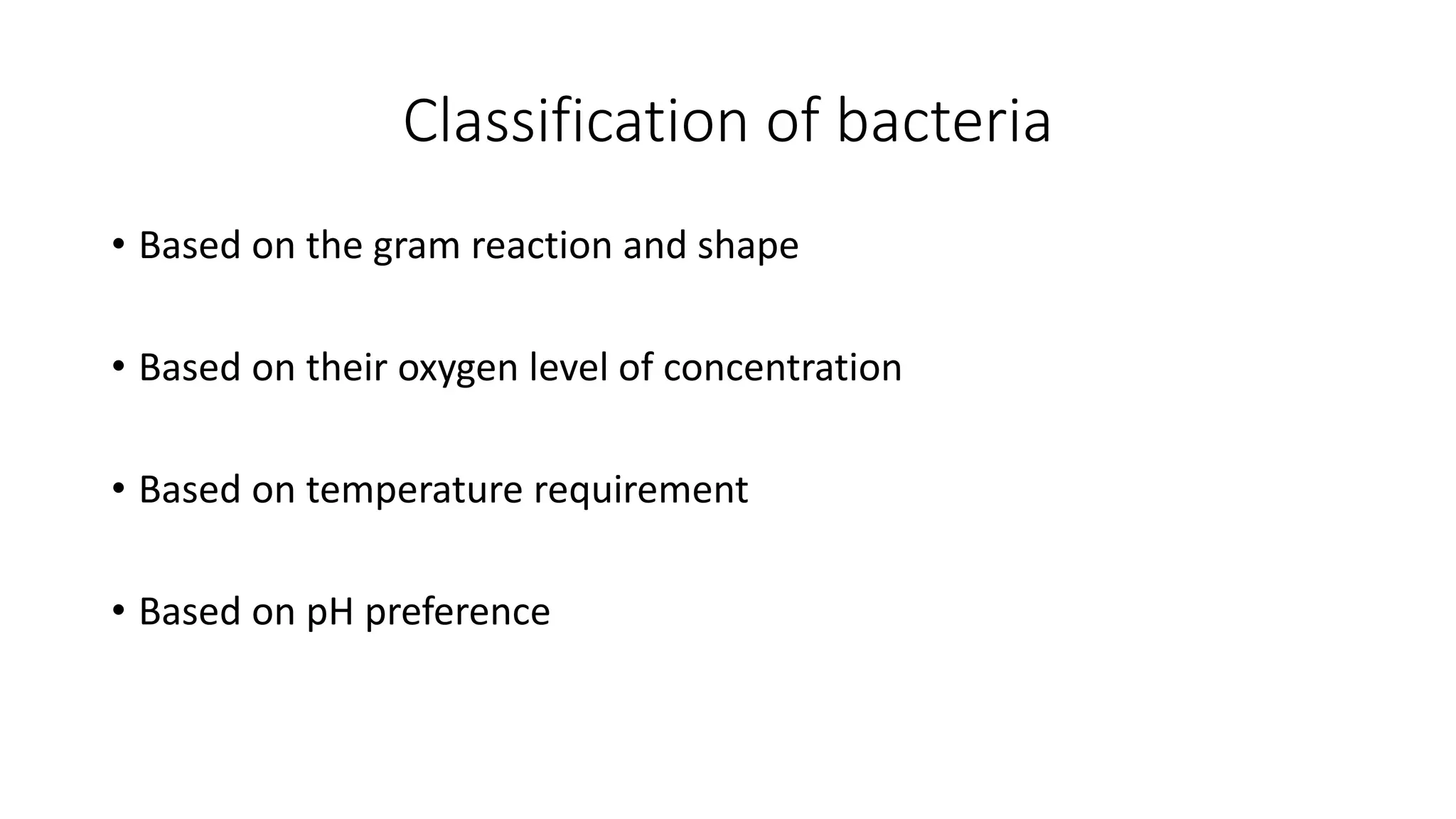
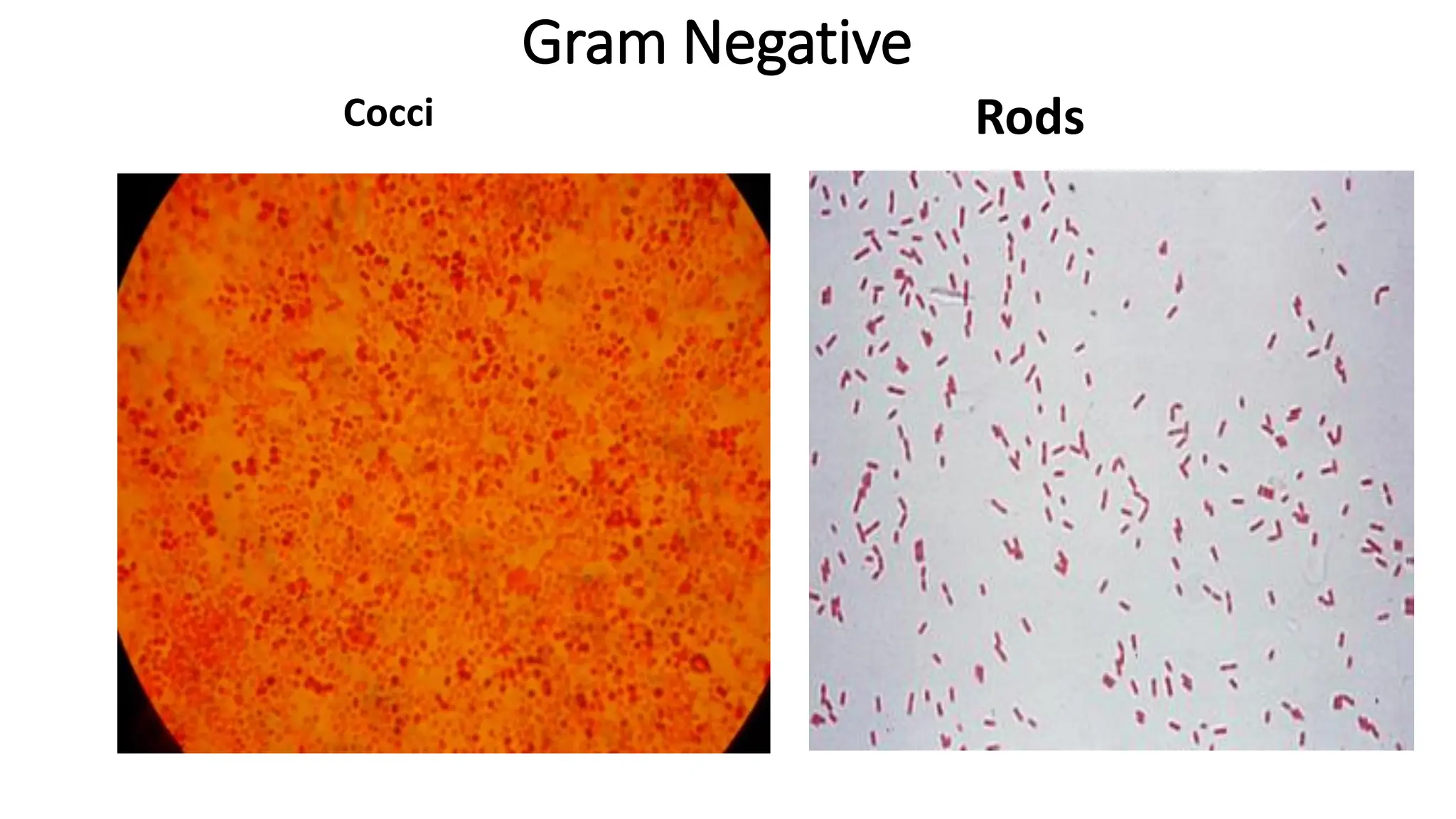
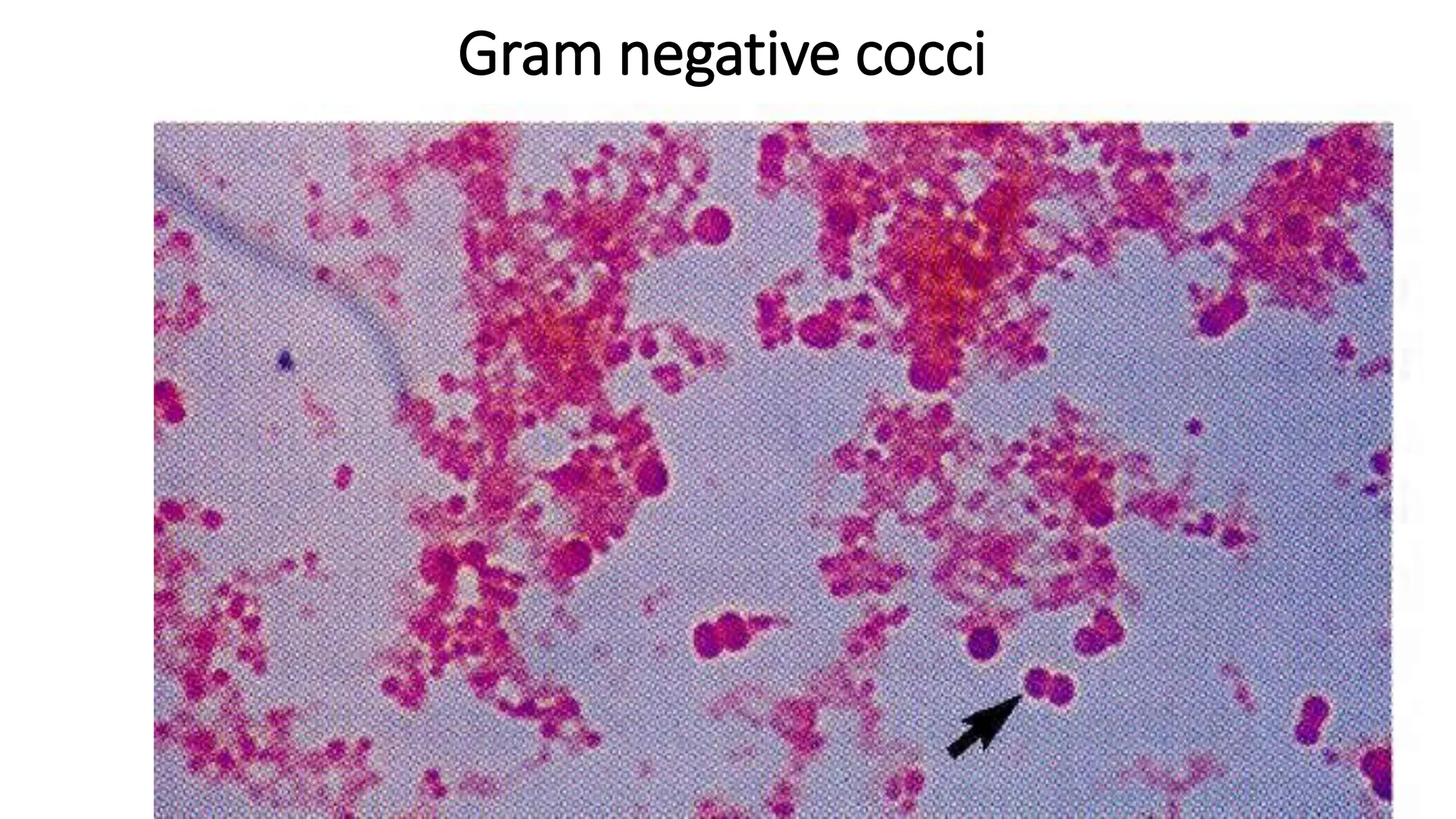
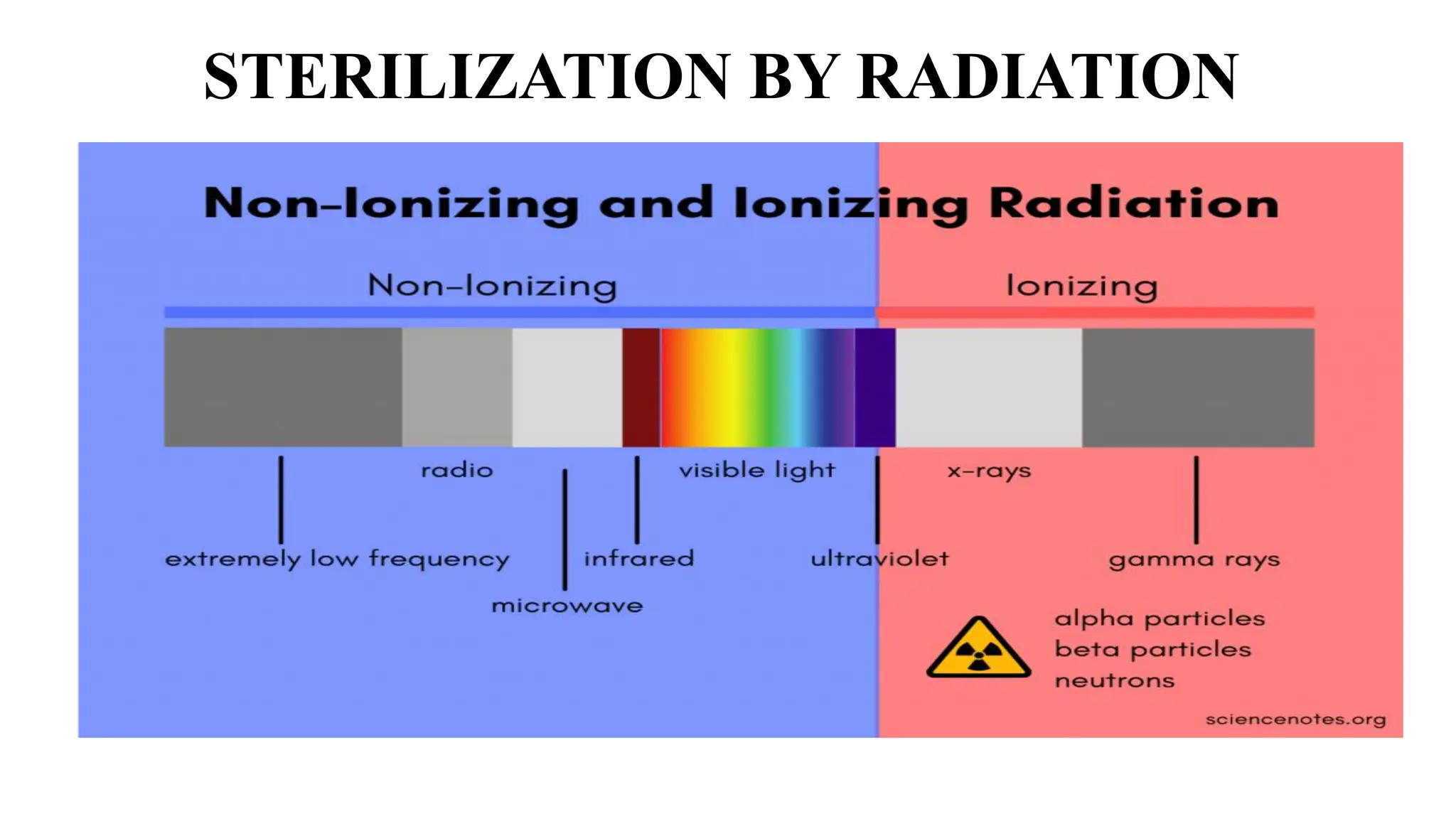
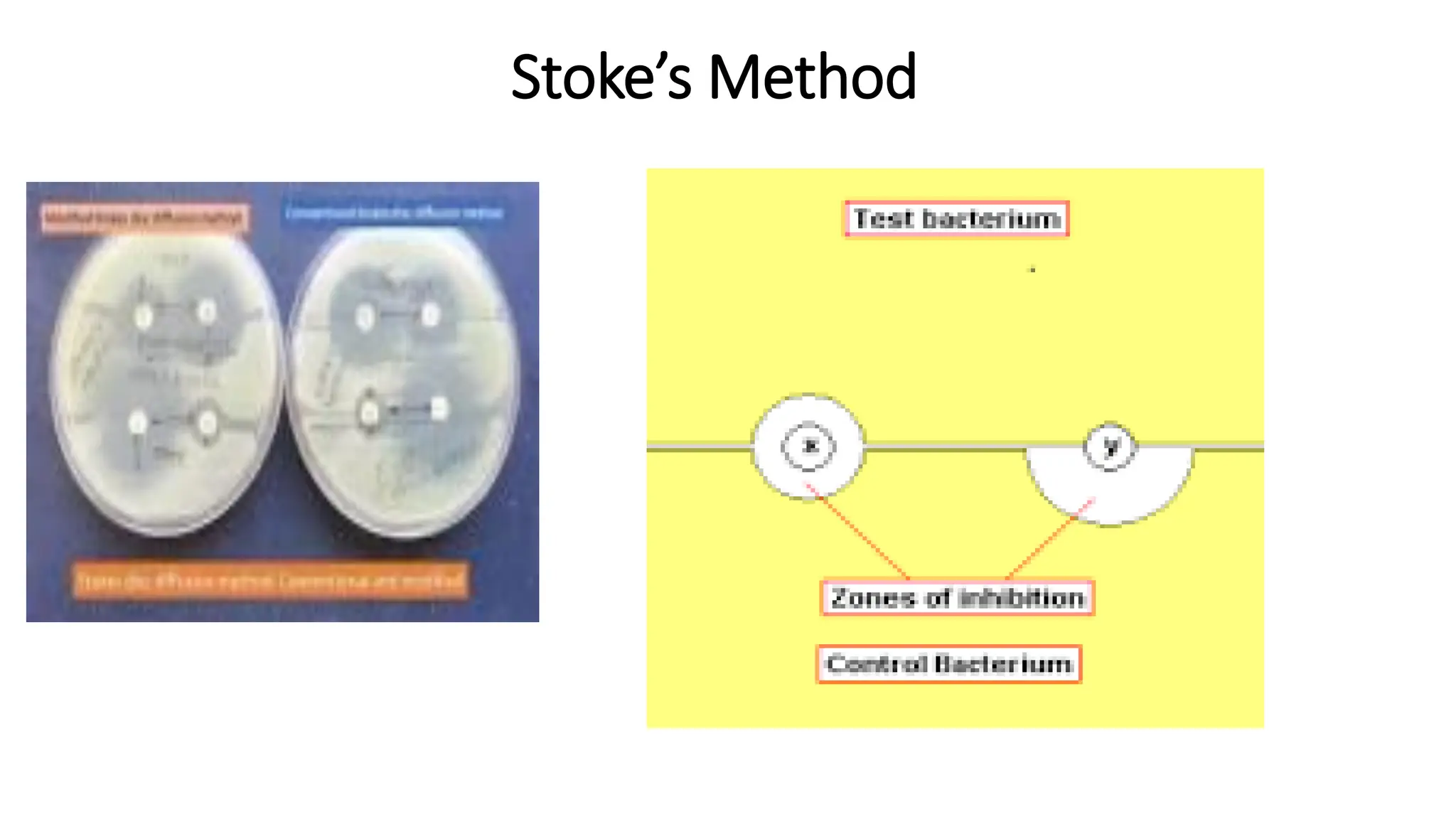
The document outlines common viruses, fungi, bacteria, and parasites along with the diseases they cause. It details clinical microbiology tools and equipment, including microscopes, autoclaves, and incubators, as well as various sterilization methods. Additionally, it discusses different media types for bacterial culture and emphasizes the importance of sterilization and disinfection in clinical settings.