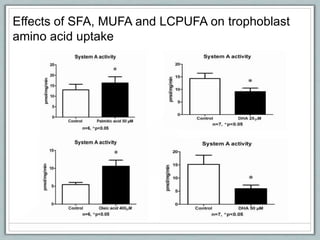
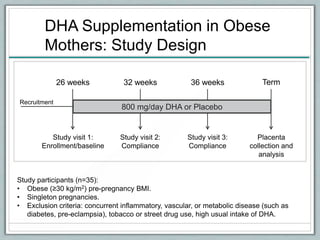
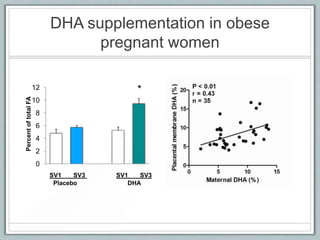
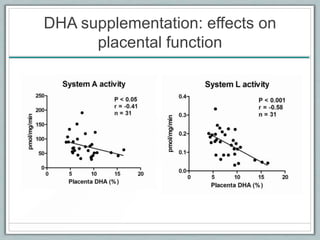
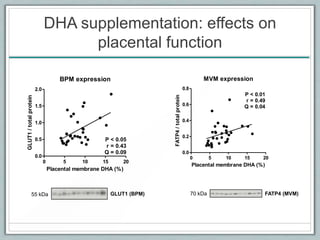
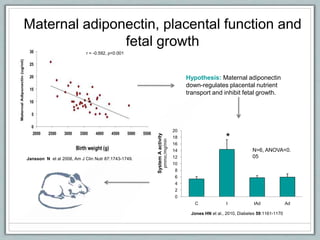
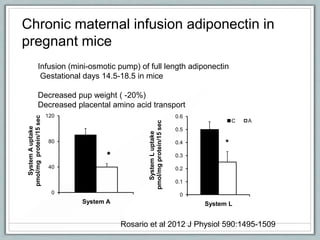
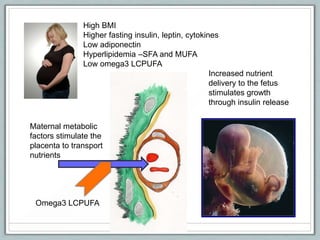
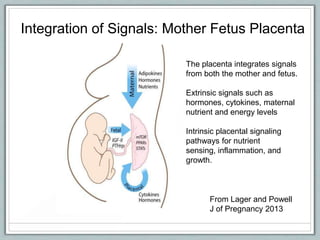
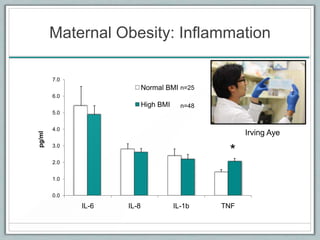
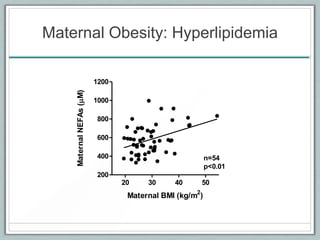
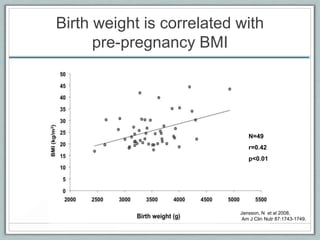
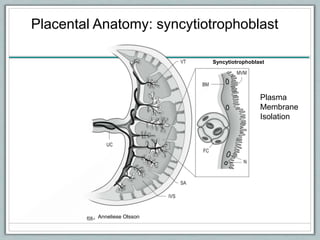
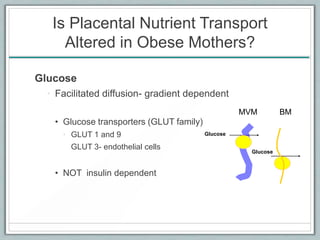
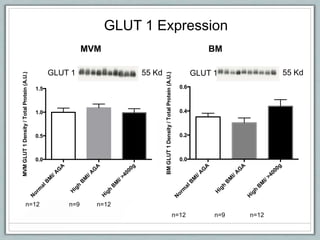
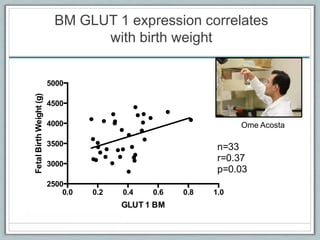
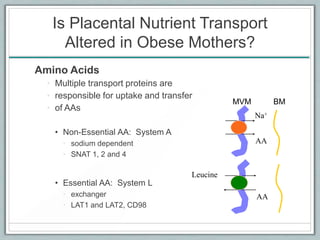
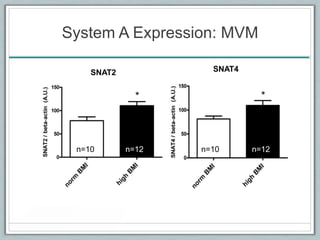
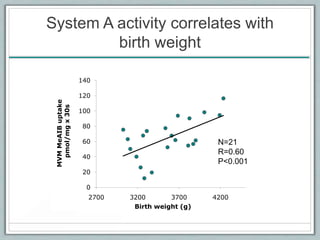
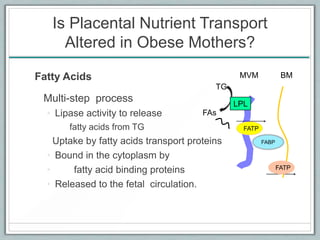
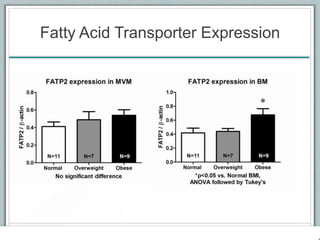
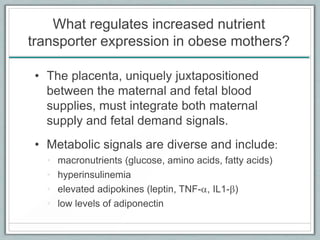
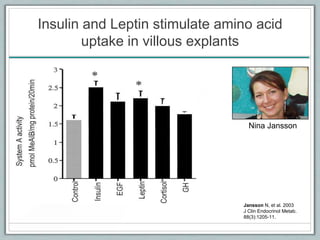
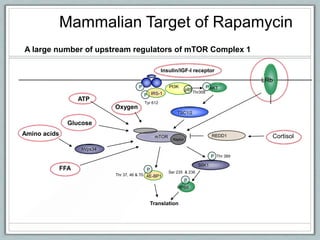
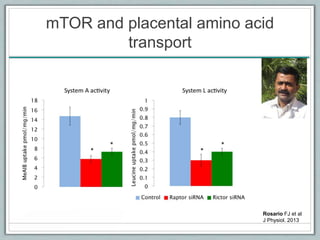
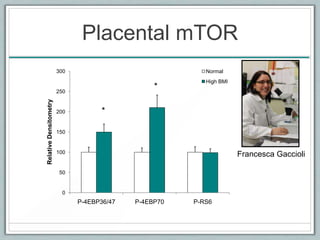
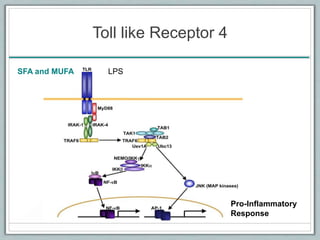
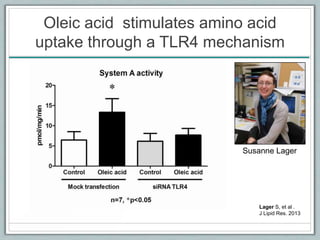
Maternal obesity is associated with increased risks during pregnancy such as gestational diabetes and fetal overgrowth. This document summarizes recent research investigating how maternal obesity impacts placental function and fetal growth. Studies presented show that in obese mothers, the placenta exhibits increased expression of nutrient transporters for glucose, amino acids, and fatty acids. This enhanced placental transport is believed to stimulate fetal growth through increased delivery of nutrients to the fetus. Factors involved in regulating these placental changes include maternal hormones, cytokines, and nutrients. Understanding these pathways may help address health issues linked to abnormal fetal growth.






![0
1
2
3
4
5
6
0 0.002 0.02 0.2
[TNF-a] ng/ml
MeAIBuptakepmol/mg/min
ANOVA p=0.004 n = 6 for each concentration
**
**
Amino acid uptake is stimulated by TNF-a
in primary cultured trophoblast cells
TNF-a
Jones et al, 2009
Am J Physiol, Cell Physiol
297:1228-1235.
Helen Jones](https://image.slidesharecdn.com/11theresapowell-130529084730-phpapp02/85/Powell-o-p2013-29-320.jpg)