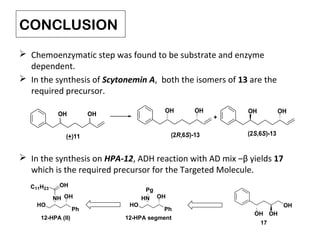
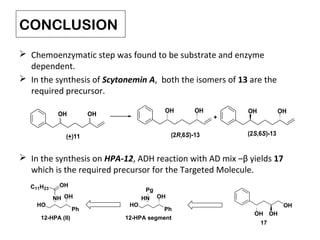
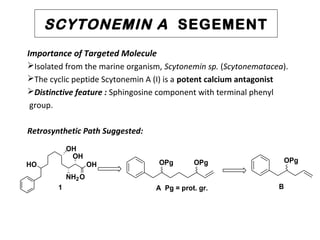
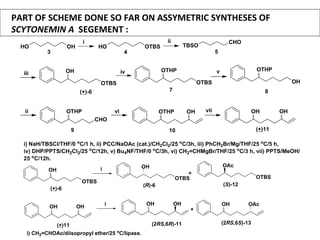
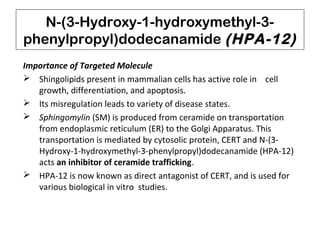
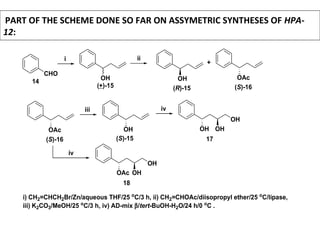
This document describes chemoenzymatic syntheses of segments of scytonemin A and HPA-12. Lipase-catalyzed kinetic resolutions were used to obtain enantiomerically pure secondary alcohols. Both (2R,6S)-13 and (2S,6S)-13 were obtained as precursors for scytonemin A synthesis. For HPA-12, alcohol dehydrogenase reaction of (S)-15 yielded (S)-17, the required precursor. Chemoenzymatic steps produced varying enantiomeric excesses depending on substrate structure and lipase used.

![CHARACTERIZATIO
N
[α]D
25
-5.4206 (c 1.07, CHCl3); 1
H NMR: δ 1.24-1.53 (m, 6H), 2.05 (s, 3H), 2.58-2.69 (m,
1H), 2.76-2.86 (m, 1H), 3.77-3.80 (m, 1H), 5.13-5.26 (m, 3H), 5.67-5.85 (m, 1H),
7.18-7.34 (m, 5H); 13
C NMR: δ 21.0, 33.8, 36.1, 43.8, 72.1, 74.5, 116.4, 126.1,
128.2, 129.2, 136.2, 138.4, 170.2.
OH OAc
(2RS,6S)-13
Scytonemin A
HPA-12
OAc
(S)-16](https://image.slidesharecdn.com/3f106958-e6df-4c01-a8a6-81c95e95a188-160621073652/85/Poster-PRESENTATION-8-320.jpg)