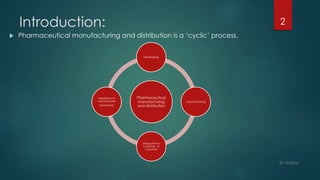
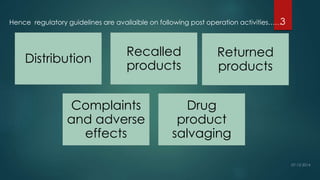
The document outlines post-operational activities in pharmaceutical manufacturing, focusing on distribution, recalls, returns, and handling complaints. It emphasizes the importance of maintaining records for product distribution and establishing protocols for managing recalls and returns due to quality issues. Regulatory guidelines are provided for ensuring compliance and safeguarding product quality throughout these processes.