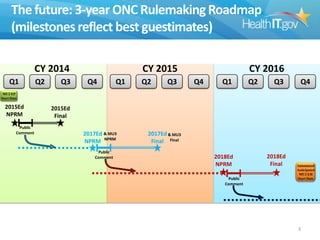
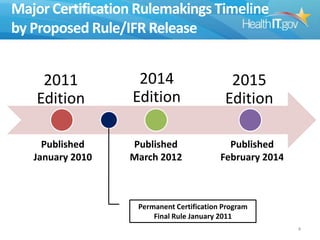
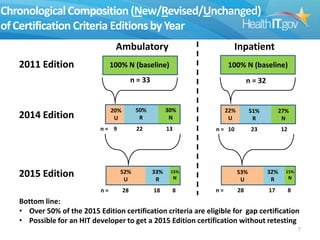
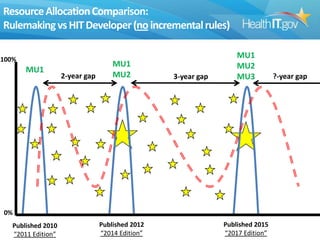
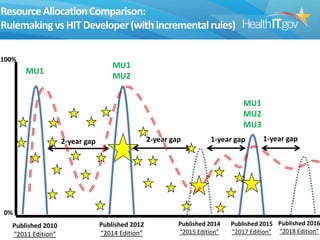
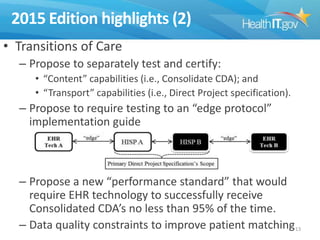
The document proposes new 2015 Edition certification criteria for electronic health records (EHRs) to support continued progress on interoperability. It highlights proposals for new and revised criteria in areas like laboratory test results, clinical decision support, implantable medical devices, care transitions, and quality reporting. The document argues that more incremental rulemaking allows for nimbler policy, reduces burden on EHR developers, and better aligns regulations with evolving industry standards and needs. Public comment is sought on the 2015 Edition proposals as well as potential 2017 Edition criteria.