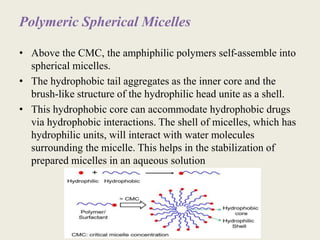
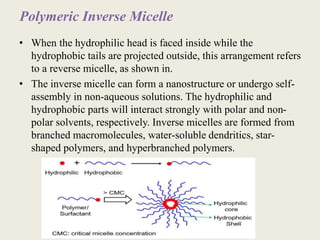
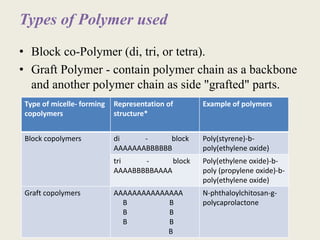
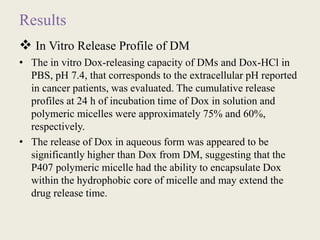
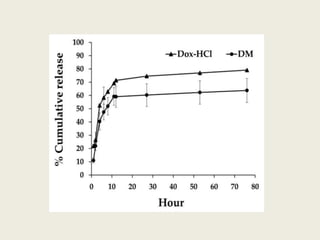
This document discusses polymeric micelles for drug delivery applications. It explains that amphiphilic polymers can self-assemble above a critical micelle concentration to form micelles with a hydrophobic core and hydrophilic shell. Doxorubicin-loaded polymeric micelles were developed using poloxamer 407, with sizes around 23 nm and high drug encapsulation. The micelles were modified to attach two FLT3 peptides, CKR and EVQ, to target leukemic stem cells and increase cytotoxicity compared to free doxorubicin. These peptide-conjugated polymeric micelles show potential as a targeted drug delivery system for acute myeloid leukemia treatment.





![Preparation of DMs
• The amount of Dox-HCl was set at 0.5 mg while the amount of P407 was
varied in many conditions to optimize the most suitable formulation for
Dox-micelle synthesis. To produce Dox encapsulated polymeric micelles, a
film-hydration technique in cooperation with a pH-induced self-assembly
method was performed [39]. In brief, poloxamer P407 was dissolved in
methanol for a final concentration of 20 mg/mL.
• The blank micelle-films were formed by evaporation under reduced
pressure in a rotary evaporator at 45 ◦C, 50 rpm, for 20 min, and then dried
at room temperature (RT) overnight. To form DMs, the blank micelle-film
was resuspended in normal saline solution (NSS) and stirred at 350 rpm for
30 min at RT. Afterwards, 10×PBS at pH 7.4 and doxorubicin aqueous
solution (Dox-HCl) were dropped into the micelle solution at the rate of
200 µL/min while stirring.
• The final volume ratio for NSS:10×PBS:Dox-HCl was 7:1:2. Following
overnight stirring, the DM solution was centrifuged at 12,000 rpm for 10
min, and then filtered through a 0.22 mm filter to obtain DMs. All samples
were stored in light-protecting containers.](https://image.slidesharecdn.com/polymericmicelles-230702052559-0658fcc7/85/Polymeric-Micelles-pptx-15-320.jpg)
![References
• Marras, A.E.; Ting, J.M.; Stevens, K.C.; Tirrell, M.V. Advances in the structural
design of polyelectrolyte complex micelles. J. Phys. Chem. B 2021, 125, 7076–
7089. [Google Scholar] [CrossRef] [PubMed]
• Dewald, I.; Fery, A. Polymeric micelles and vesicles in polyelectrolyte multilayers:
Introducing hierarchy and compartmentalization. Adv. Mater. Interfaces 2017, 4,
1600317. [Google Scholar] [CrossRef]
• Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973.
[Google Scholar] [CrossRef] [PubMed][Green Version]
• u, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the critical micelle
concentration of surfactants by a simple and fast titration method. Anal.
Chem. 2020, 92, 4259–4265. [Google Scholar] [CrossRef]
• Su, H.; Wang, F.; Ran, W.; Zhang, W.; Dai, W.; Wang, H.; Anderson, C.F.; Wang,
Z.; Zheng, C.; Zhang, P.; et al. The role of critical micellization concentration in
efficacy and toxicity of supramolecular polymers. Proc. Natl. Acad. Sci.
USA 2020, 117, 4518–4526. [Google Scholar] [CrossRef]
• Chueahongthong, F.; Tima, S.; Chiampanichayakul, S.; Dejkriengkraikul, P.;
Okonogi, S.; Sasarom, M.; Rodwattanagul, S.; Berkland, C.; Anuchapreeda, S.
Doxorubicin-Loaded Polymeric Micelles Conjugated with CKR- and EVQ-FLT3
Peptides for Cytotoxicity in Leukemic Stem Cells. Pharmaceutics 2022, 14, 2115.
https://doi.org/10.3390/ pharmaceutics14102115](https://image.slidesharecdn.com/polymericmicelles-230702052559-0658fcc7/85/Polymeric-Micelles-pptx-20-320.jpg)
