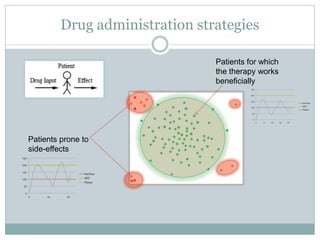
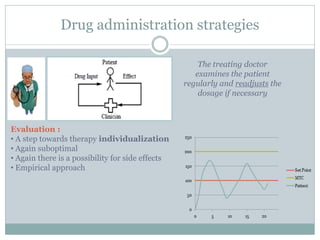
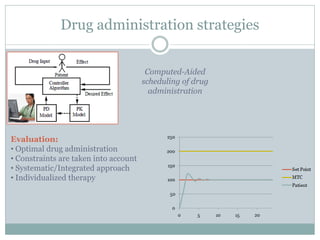
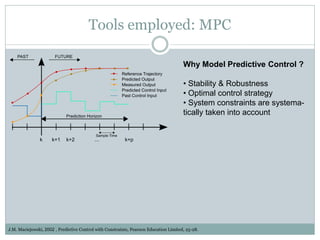
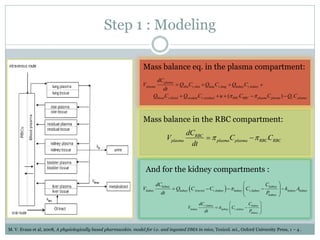
This document describes a physiologically based pharmacokinetic (PBPK) modeling and model predictive control (MPC) approach for optimal drug administration. It involves 4 main steps:
1) Developing PBPK models using mass balance equations to describe drug distribution in compartments like plasma, red blood cells, and kidneys.
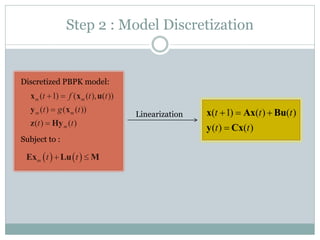
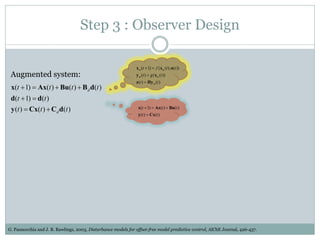
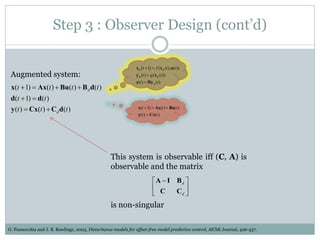
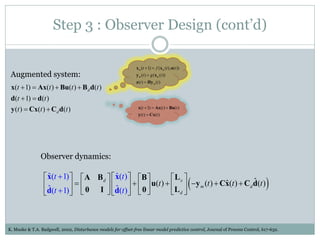
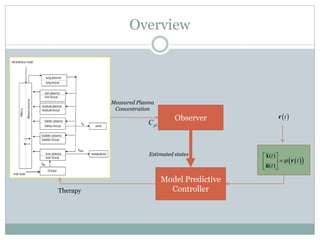
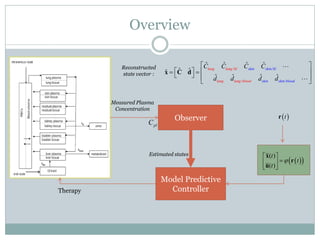
2) Discretizing the PBPK models and designing an observer to estimate unmeasured states using the measured plasma concentration.
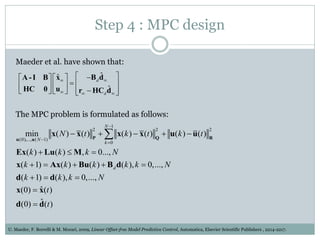
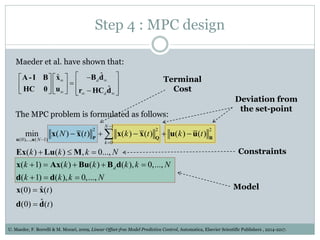
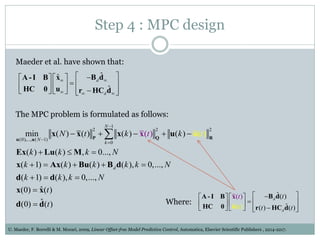
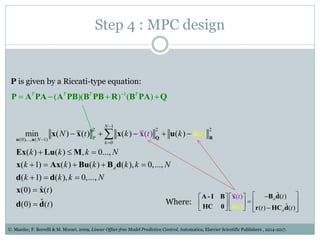
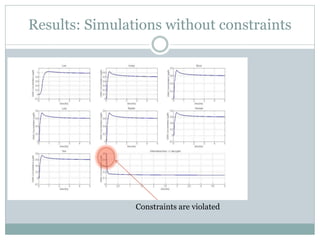
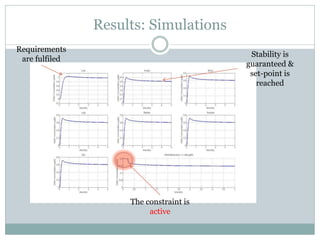
3) Formulating an MPC problem to determine optimal drug administration inputs over a prediction horizon while satisfying constraints like toxicity limits.
4) Solving the MPC problem online to determine the optimal control action and adjust the drug dosage based on the