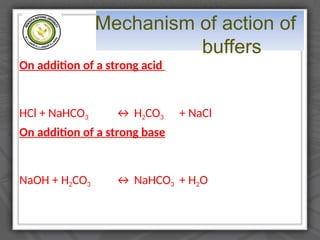
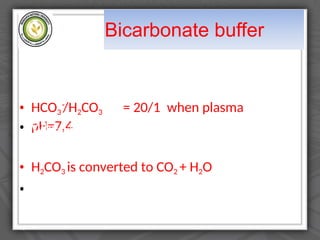
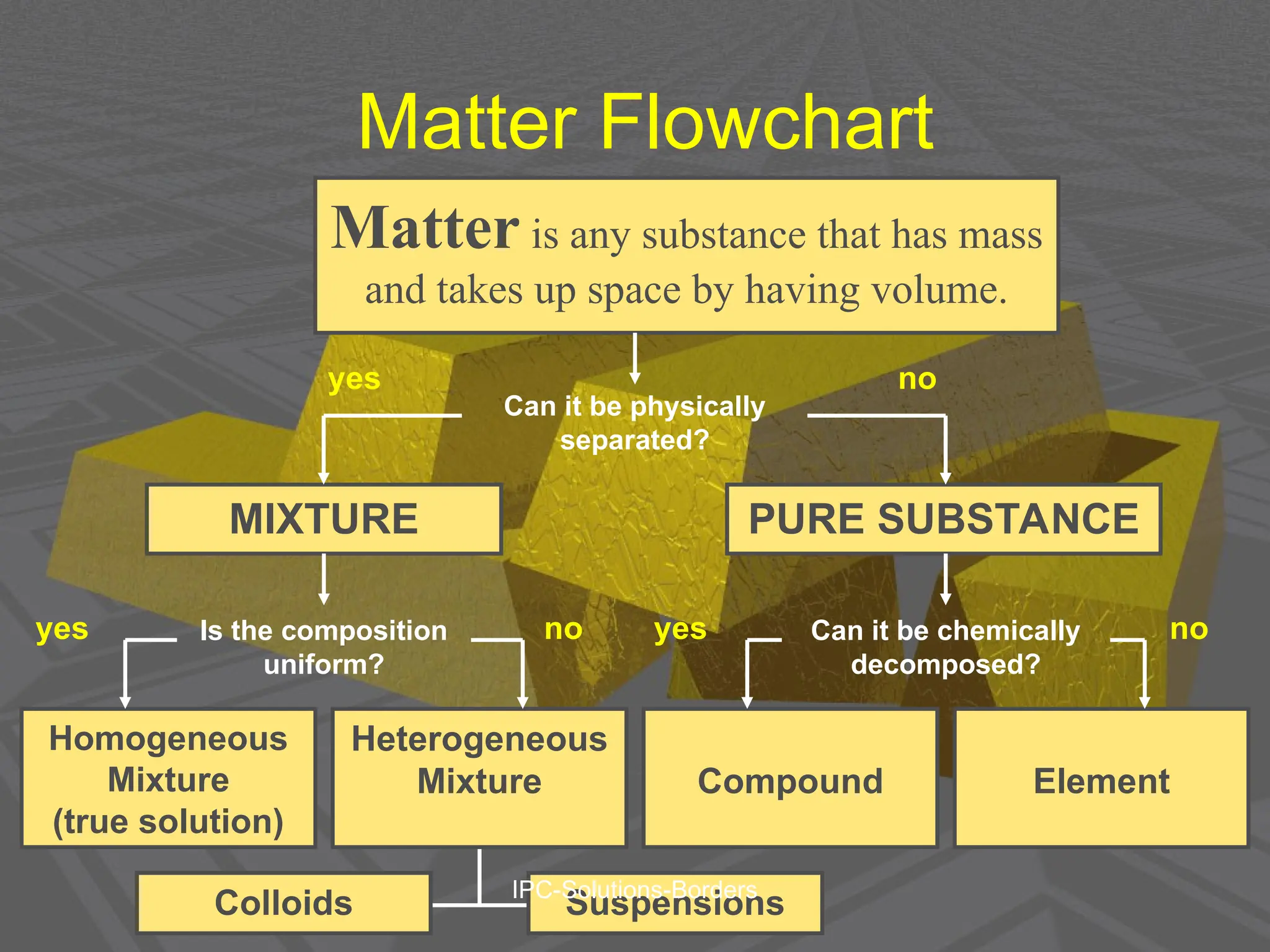
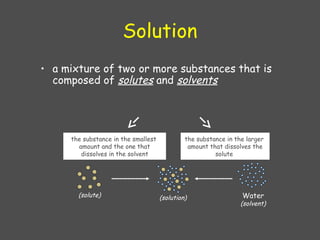
The document explains the classification of matter and mixtures, including true solutions, colloidal solutions, and suspensions, along with their characteristics. It details the concept of pH and describes acids and bases as proton donors and acceptors, respectively. Additionally, it discusses buffer solutions, their mechanism of action, and the physiological buffer systems that help maintain pH stability in the body.



![pH
• pH is commonly expressed as –log[H+]
• the negative log (base10) of the
concentrations of hydrogen ions H+ in solution
• The pH scale](https://image.slidesharecdn.com/physicalchemistry-241118220538-9d0807bb/85/physical-chemistry-and-buffer-system-ppt-6-320.jpg)