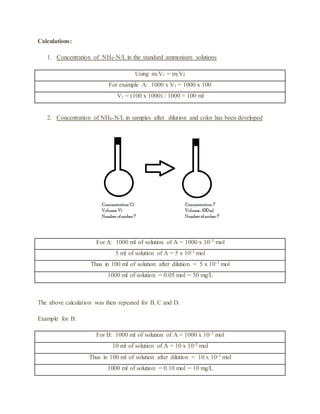
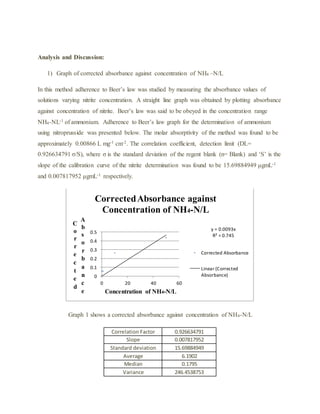
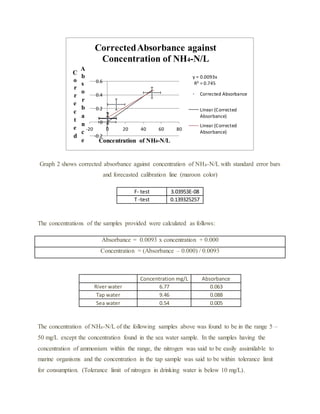
The document details an experiment using the manual phenate method to determine the concentration of ammonium in various water samples through spectrophotometric analysis. It outlines the procedure for preparing reagents and standard solutions, along with necessary safety precautions and equipment. The results indicate the method's effectiveness in measuring ammonium levels, adhering to Beer’s law, and its application in industrial and pharmaceutical contexts.