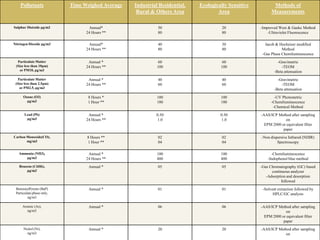
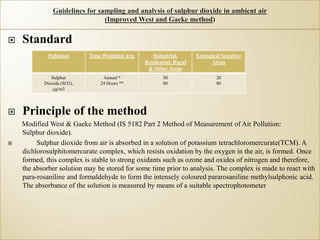
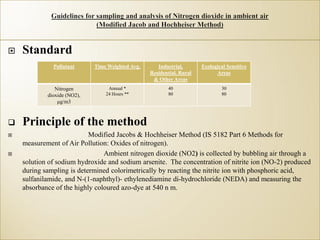
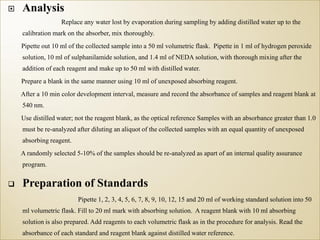
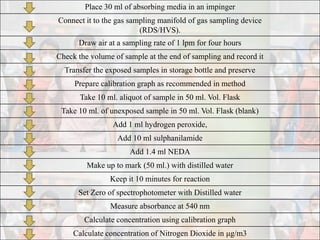
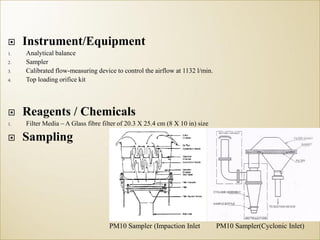
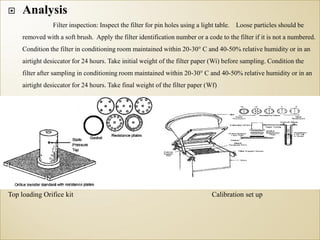
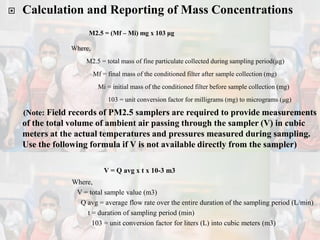
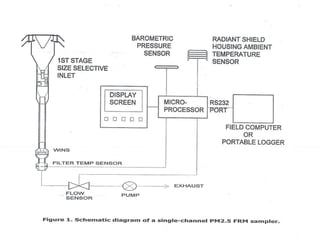
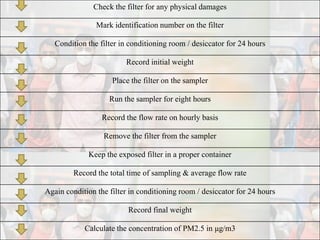
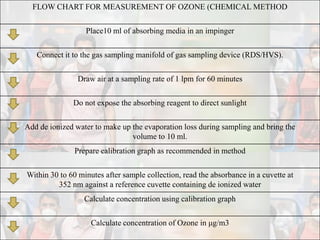
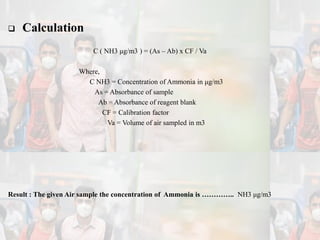
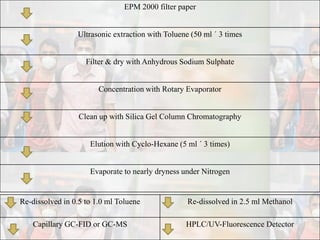
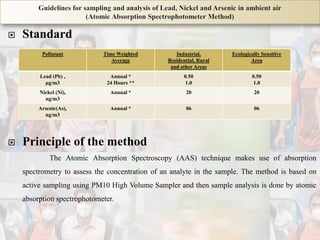
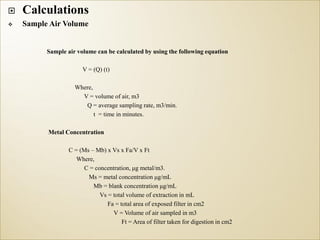
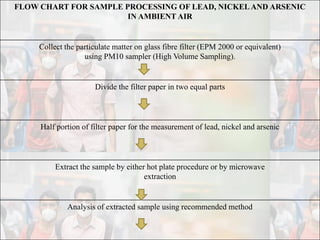
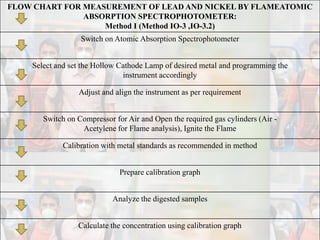
1) Guidelines were notified for sampling and analysis of common air pollutants to have uniform monitoring across India and allow for comparison of data.
2) The methods prescribed are a combination of physical, wet-chemical, and continuous online methods to meet National Ambient Air Quality Standards.
3) Accurate monitoring requires a combination of manual and continuous methods at each location as well as proper laboratory infrastructure.