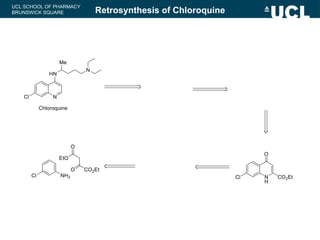
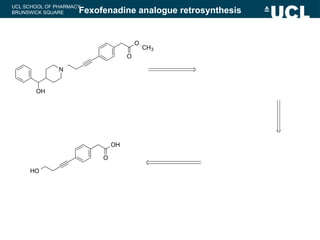
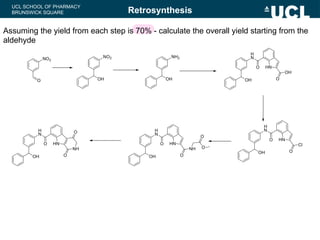
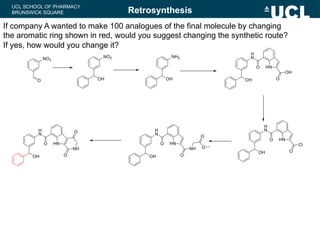
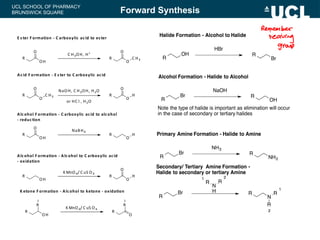
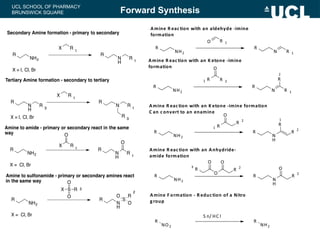
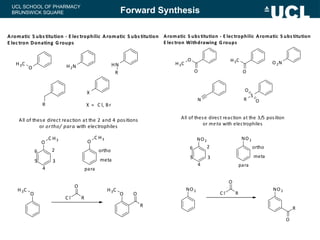
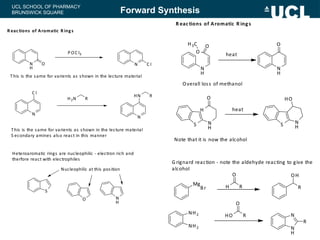
This document summarizes the key steps in a chemical synthesis workshop, including forward synthesis reactions such as converting a halide to an alcohol, primary amine, or secondary/tertiary amine. It also covers retrosynthesis techniques like disconnecting molecules in 1 or 2 steps to outline possible synthesis routes, and examples of retrosynthesizing specific compounds like chloroquine and a fexofenadine analogue. The document emphasizes planning synthesis routes strategically and calculating overall yields from multiple steps.


![UCL SCHOOL OF PHARMACY
BRUNSWICK SQUARE
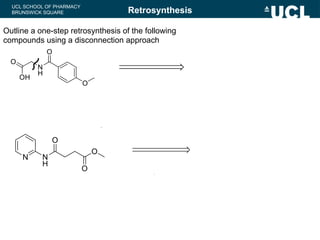
Outline a one-step retrosynthesis of the following
compound using a disconnection approach
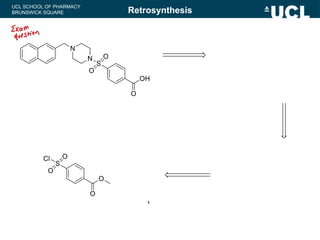
Retrosynthesis
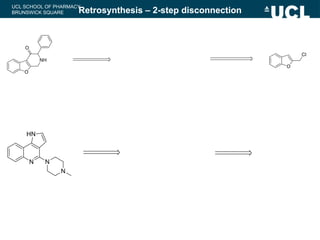
Outline a two-step retrosynthesis of the following
compound using a disconnection approach
OH
= 0 ] equivalence](https://image.slidesharecdn.com/phay0006chemicalsynthesisworkshop2-220828140056-60392b5c/85/PHAY0006-Chemical-Synthesis-workshop-2-pdf-8-320.jpg)