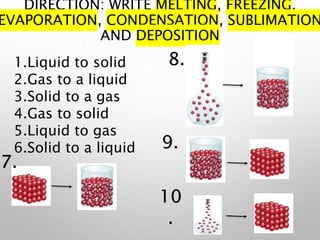
This document discusses the three phases of matter - solid, liquid, and gas - and the phase changes that occur between them. It explains that phase changes require the absorption or release of heat energy as molecules speed up or slow down and move farther apart or closer together. The document then defines and provides examples of different types of phase changes: melting (solid to liquid), freezing (liquid to solid), evaporation (liquid to gas), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid).