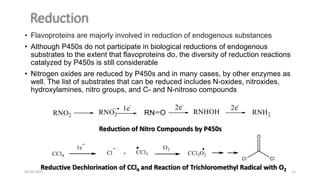
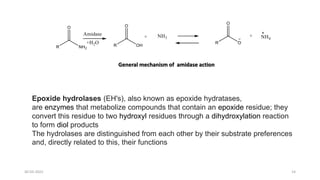
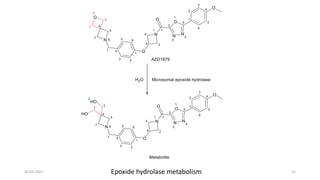
Phase I drug metabolism involves introducing reactive or polar groups into drugs through specialized enzyme systems like the cytochrome P450 system. This helps determine a drug's effects, understand drug interactions, and allows for prodrug synthesis. Phase I reactions like oxidation and hydrolysis are carried out by cytochrome P450 and mixed function oxidases in the liver. Cytochrome P450 enzymes catalyze reactions like hydroxylation that can activate prodrugs or make drugs more toxic. Phase I metabolites may be excreted or undergo further Phase II conjugation reactions.