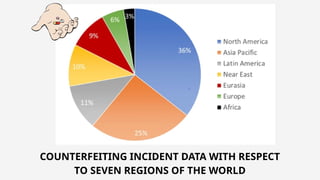
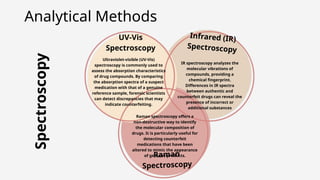
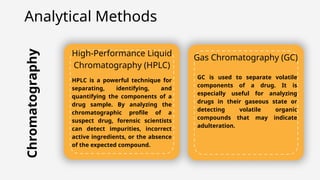
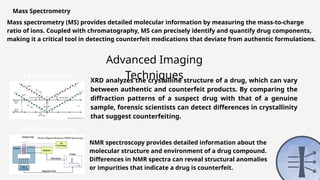
The document discusses pharmaceutical counterfeiting, which involves the production of mislabeled medications posing significant public health risks, particularly in low- and middle-income countries. It highlights the critical role of forensic investigation in detecting counterfeits using techniques like spectroscopy and chromatography, alongside legal frameworks and the responsibilities of pharmacists and pharmaceutical companies. Case studies emphasize the impact of counterfeit drugs and the necessity for stringent regulatory measures and public awareness to combat this pervasive issue.