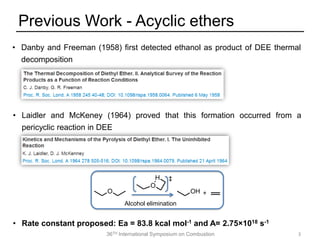
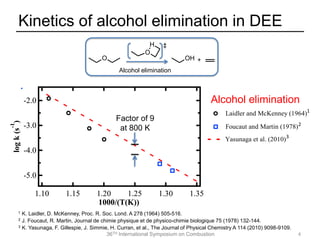
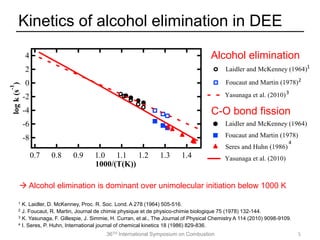
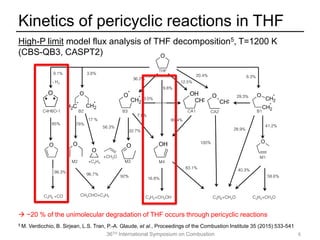
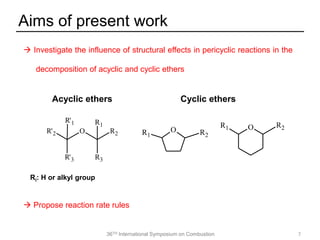
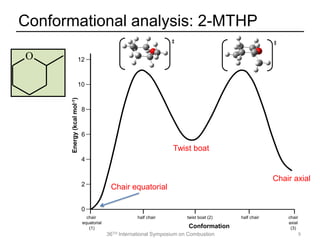
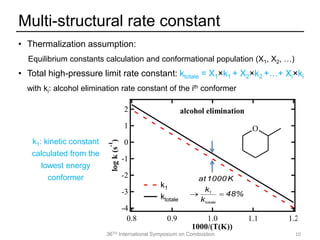
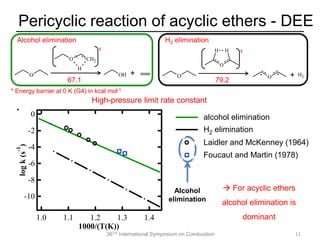
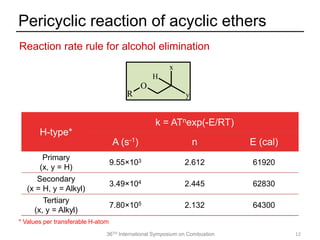
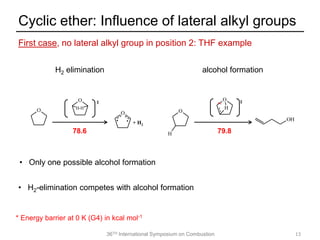
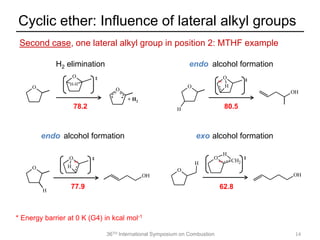
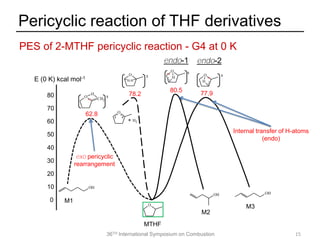
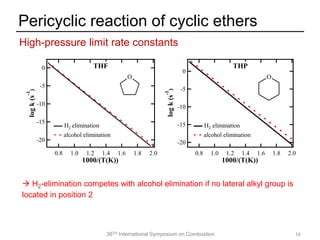
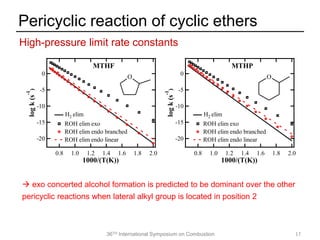
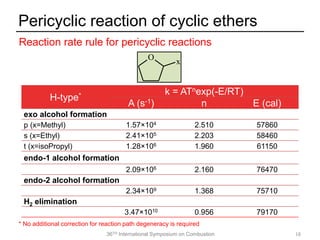
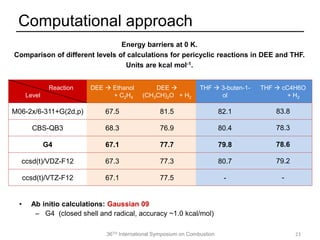
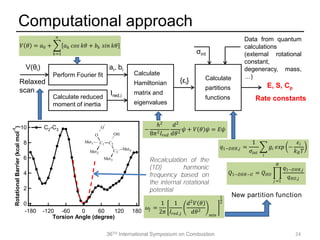
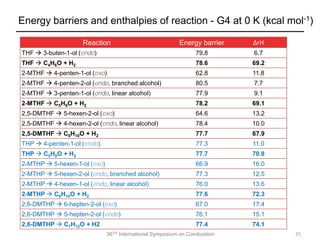
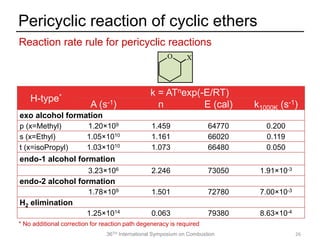
The document discusses pericyclic reactions in acyclic and cyclic ethers as biofuels, focusing on their combustion behaviors and reaction mechanisms. It highlights that alcohol elimination is dominant in acyclic ethers, while cyclic ethers exhibit more complex behaviors influenced by lateral alkyl groups. The study utilizes quantum chemical calculations to propose reaction rate rules and investigates the structural effects on the decomposition of these ethers.