Patient with Fluid, Electrolyte, and Burn Management – Nursing Lecture Notes & Presentation
This SlideShare presentation offers a comprehensive and visually organized summary of key concepts related to fluid and electrolyte balance and burn injury management in nursing care. Perfect for BSN, Post-RN, and diploma nursing students, as well as for revision, class presentations, or exam prep.
📚 Contents Include:
Classification and severity of burns
Pathophysiology of fluid loss in burn patients
Electrolyte disturbances and their clinical manifestations
Emergency burn care: Airway, Breathing, Circulation (ABC)
Fluid resuscitation strategies (including Parkland Formula)
Long-term management: skin grafts, infection control, nutrition
Nursing responsibilities in burn units and ICU settings
Charts, images, and flow diagrams for quick retention
👩⚕️ Designed for:
BSc Nursing / Post-RN Nursing Students
Nurse educators and trainers
NCLEX-RN, PN, and local licensing exam candidates
Clinical practice enhancement









![Pathophysiology Post-RN BSN Note Chapter 05 Patient With Fluid Electrolyte Burn
Page 10 of 26
➢ While sodium [Na+] and potassium [K+] rely on transport mechanisms such as the Na+/K+ pump
located in the cell membrane for movement across the membrane. Because the Na+/K+ pump
relies on adenosine triphosphate (ATP) and the enzyme ATPase for energy, it is often referred to as
the Na+/K+-ATPase membrane pump.
➢ Water crosses the cell membrane by osmosis using special transmembrane protein channels that
are called aquaporin
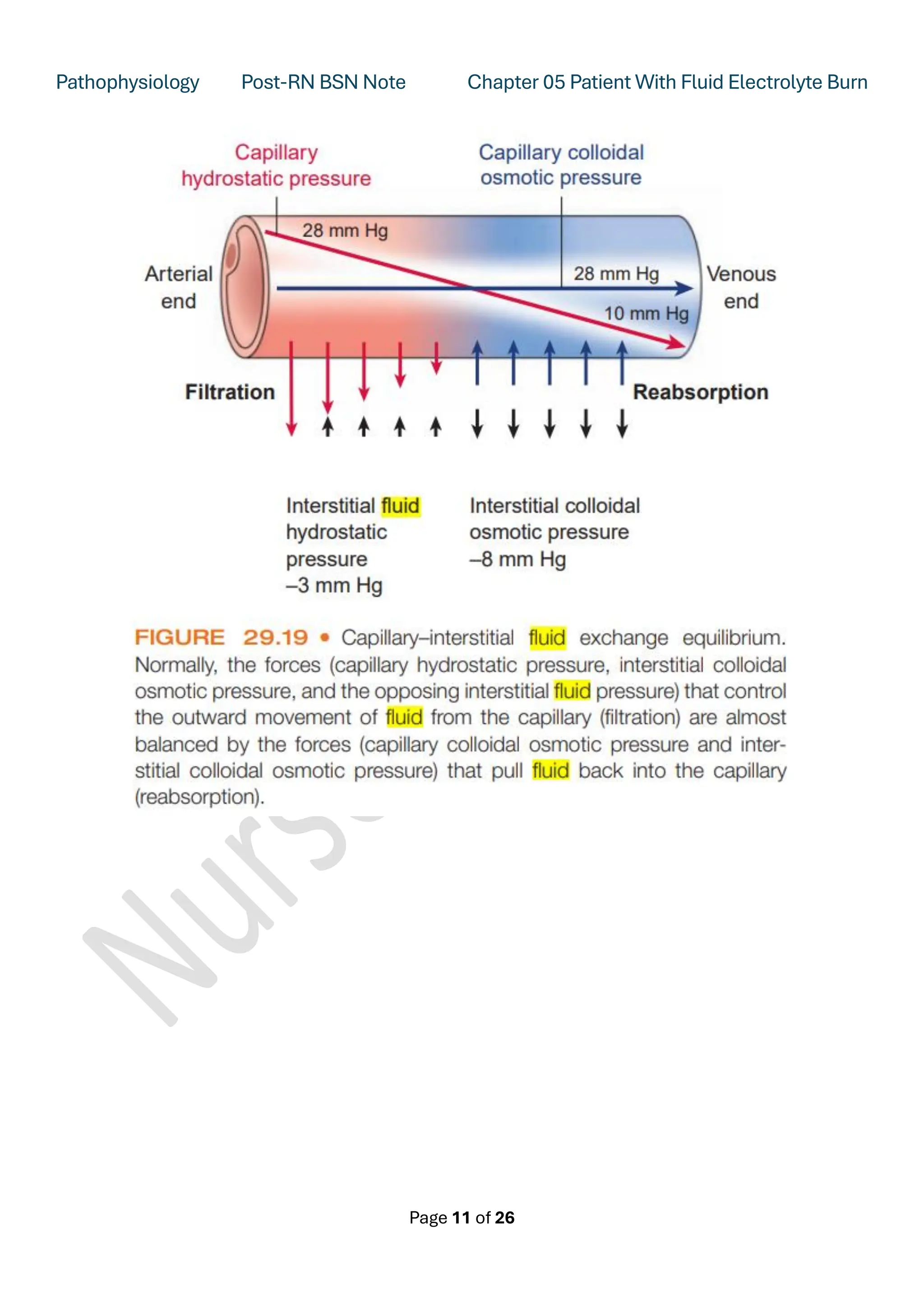
Starling's Hypothesis
Starling's hypothesis states that the fluid movement due to filtration across the wall of a capillary is
dependent on the balance between the hydrostatic pressure gradient and the oncotic pressure
gradient across the capillary.
The four Starling's forces are:
1. Hydrostatic pressure in the capillary (pc)
2. Hydrostatic pressure in the interstitium (pi)
3. Oncotic pressure in the capillary (pc )
4. Oncotic pressure in the interstitium (pi )
➢ Normally, the movement of fluid between the capillary bed and the interstitial spaces is
continuous.
➢ As Earnest Henry Starling pointed out, a state of equilibrium exists as long as equal amounts of
fluid enter and leave the interstitial spaces. This is referred to as “Starling forces”.
➢ The hydrostatic pressure at the arterial end of the capillary is higher than at the venous end.
➢ The capillary colloidal osmotic pressure and opposing interstitial osmotic pressure determine the
reabsorption of fluid at the venous end of the capillary. A slight imbalance in forces causes slightly
more filtration of fluid into interstitial spaces than absorption back into the capillary. It is this fluid
that is returned to the circulation by the lymphatic system.](https://image.slidesharecdn.com/pathophysiologychapter5electrolyteimbalanceinburnpatientnotes-250629091805-0ffc571c/75/Pathophysiology-Chapter-5-Electrolyte-Imbalance-in-Burn-Patient-Notes-pdf-10-2048.jpg)


![Pathophysiology Post-RN BSN Note Chapter 05 Patient With Fluid Electrolyte Burn
Page 14 of 26
III- Hypertonic Solution:
A solution with an osmalality higher than that of serum.
e.g. D/S 0.9%, D/S 0.18%, D/S 0.45%, D10W, D25W.
Isotonic Imbalance
➢ Intracellular fluid volume remains constant
➢ External loss: vomiting, diarrhoea, haemorrhage, burning
➢ Internal loss: ileus, ascites, pleural effusion
➢ Therapy: volume replacement with isotonic solution
2. Osmotic imbalance
➢ As water moves across the semipermeable membrane, it generates a pressure called the osmotic
pressure.
➢ The magnitude of the osmotic pressure represents the hydrostatic pressure (measured in
millimeters of mercury [mm Hg]) needed to oppose the movement of water across the membrane.
➢ The osmotic activity that non-diffusible particles exert in pulling water from one side of the semi-
permeable membrane to the other is measured by a unit called an osmole.
➢ Osmolarity refers to the osmolar concentration in 1 L of solution (mOsm/L).
➢ Osmolality to the osmolar concentration in 1 kg of water (mOsm/kg of H2 O).](https://image.slidesharecdn.com/pathophysiologychapter5electrolyteimbalanceinburnpatientnotes-250629091805-0ffc571c/75/Pathophysiology-Chapter-5-Electrolyte-Imbalance-in-Burn-Patient-Notes-pdf-14-2048.jpg)







![Pathophysiology Post-RN BSN Note Chapter 05 Patient With Fluid Electrolyte Burn
Page 23 of 26
➢ The potassium content of the ECF (3.5 to 5 mEq/L [3.5 to 5 mmol/L]) is considerably lower.
➢ Hypokalemia refers to a decrease in plasma potassium levels below 3.5 mEq/L (3.5 mmol/L).](https://image.slidesharecdn.com/pathophysiologychapter5electrolyteimbalanceinburnpatientnotes-250629091805-0ffc571c/75/Pathophysiology-Chapter-5-Electrolyte-Imbalance-in-Burn-Patient-Notes-pdf-23-2048.jpg)


