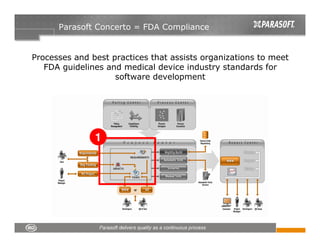
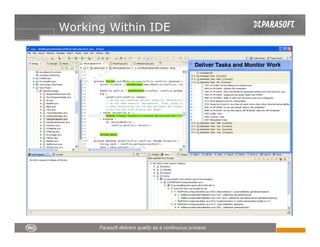
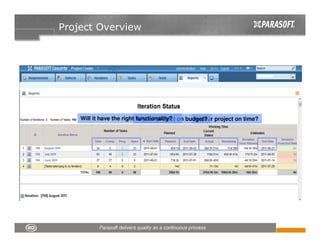
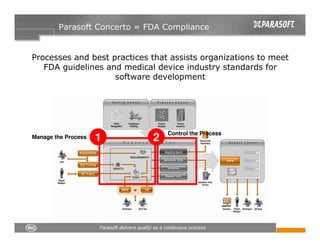
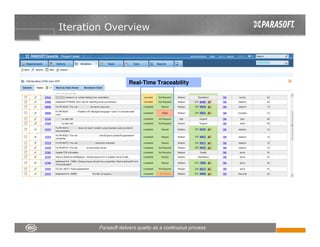
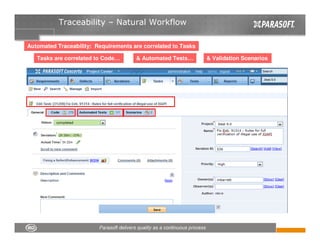
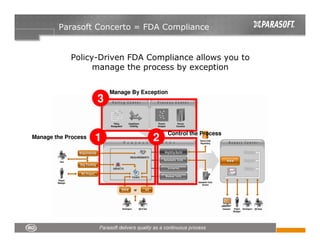
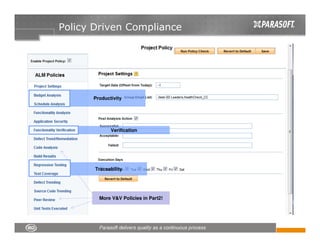
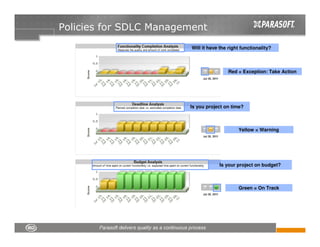
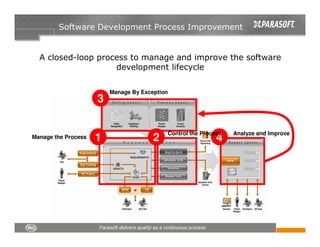
This document discusses FDA regulations for medical device software and how Parasoft Concerto can help with compliance. It notes that many medical device recalls are due to software defects introduced after release. The FDA provides guidance on software validation best practices including verification activities throughout the software development lifecycle (SDLC) and requirements traceability. Parasoft Concerto implements these practices by automating processes, providing visibility into activities, and enabling traceability between requirements, code, tests and validation scenarios. It also allows managing the software development process through policy-driven compliance checks.