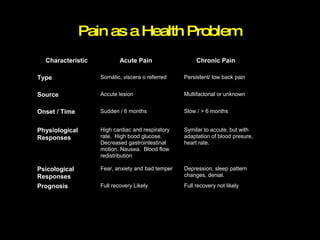
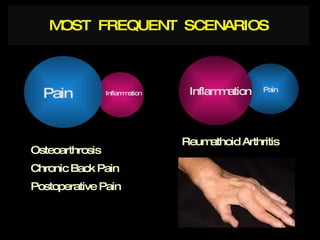
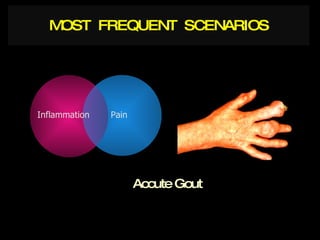
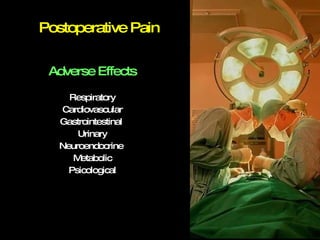
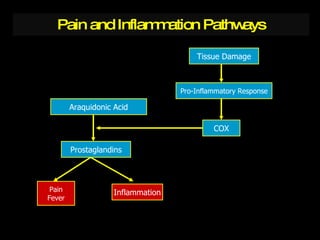
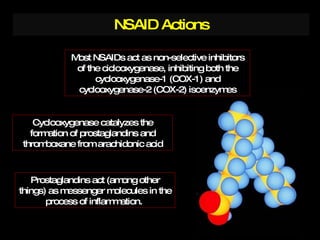
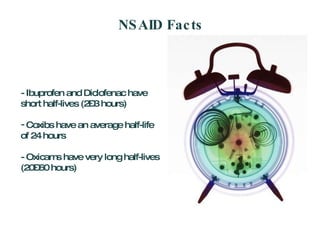
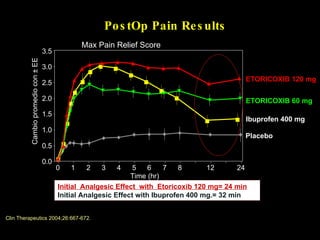
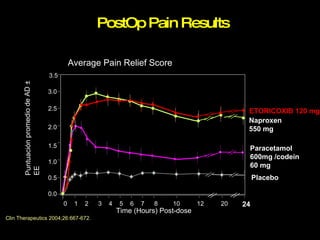
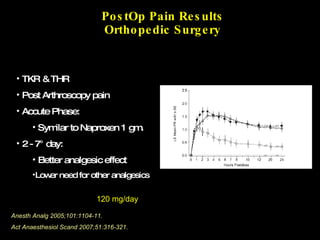
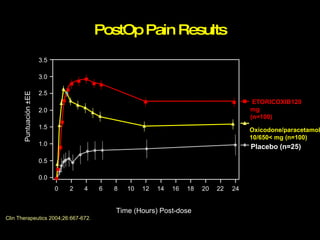
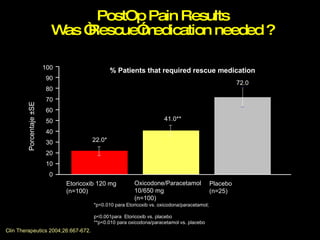
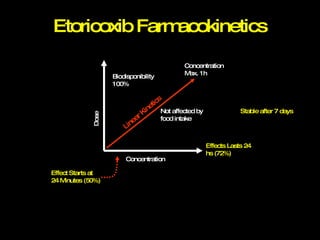
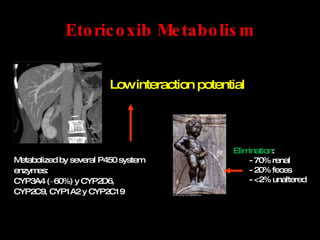
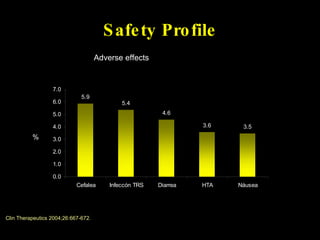
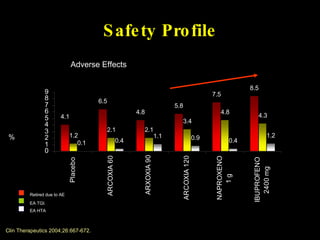
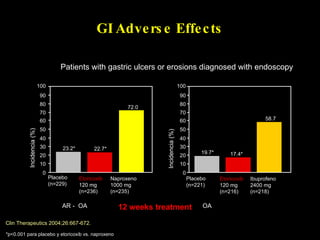
The document discusses postoperative pain management. Effective pain management has humanitarian benefits and can facilitate rapid recovery. It summarizes various pain theories and treatments, including opioids, NSAIDs, and other non-opioid analgesics. It also provides examples of etoricoxib clinical trials that demonstrate its efficacy in reducing postoperative pain with fewer side effects compared to other treatments like ibuprofen and oxycodone.