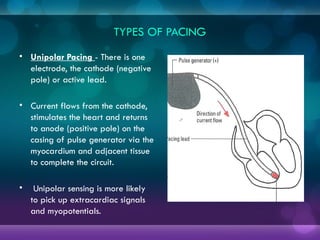
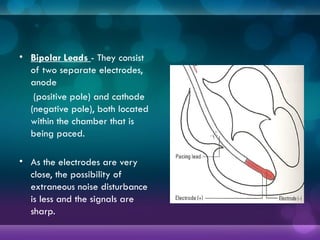
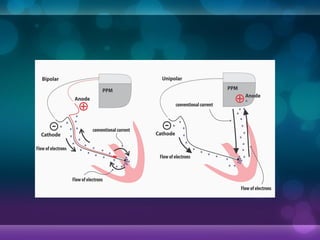
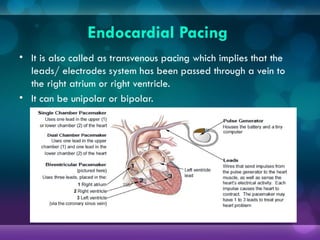
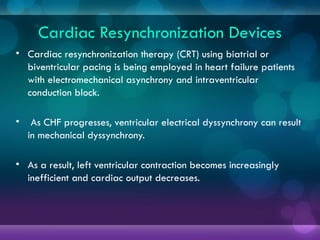
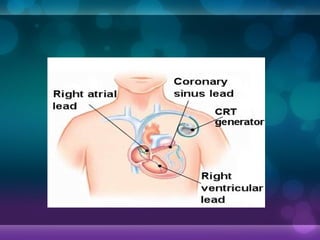
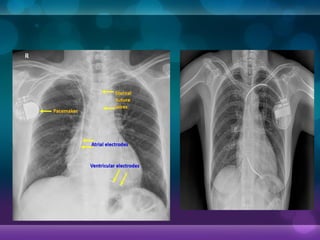
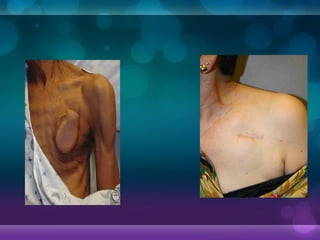
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
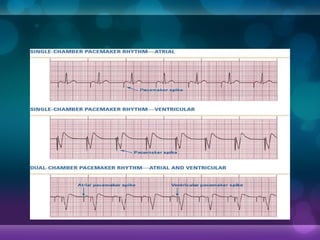
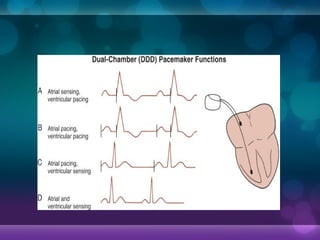
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian engineer John Hopps developed a portable pacemaker using vacuum tubes.
In 1958, Dr. Åke Senning and engineer Rune Elmqvist implanted the first fully functional pacemaker in a patient in Sweden.
Advancements (1960s-1990s)
Lithium-ion batteries replaced older mercury-zinc batteries, increasing longevity.
The first demand pacemakers, which only activate when needed
1932, Dr. Albert Hyman designed the first artificial pacemaker, though it was not widely used.
In 1950, Canadian e