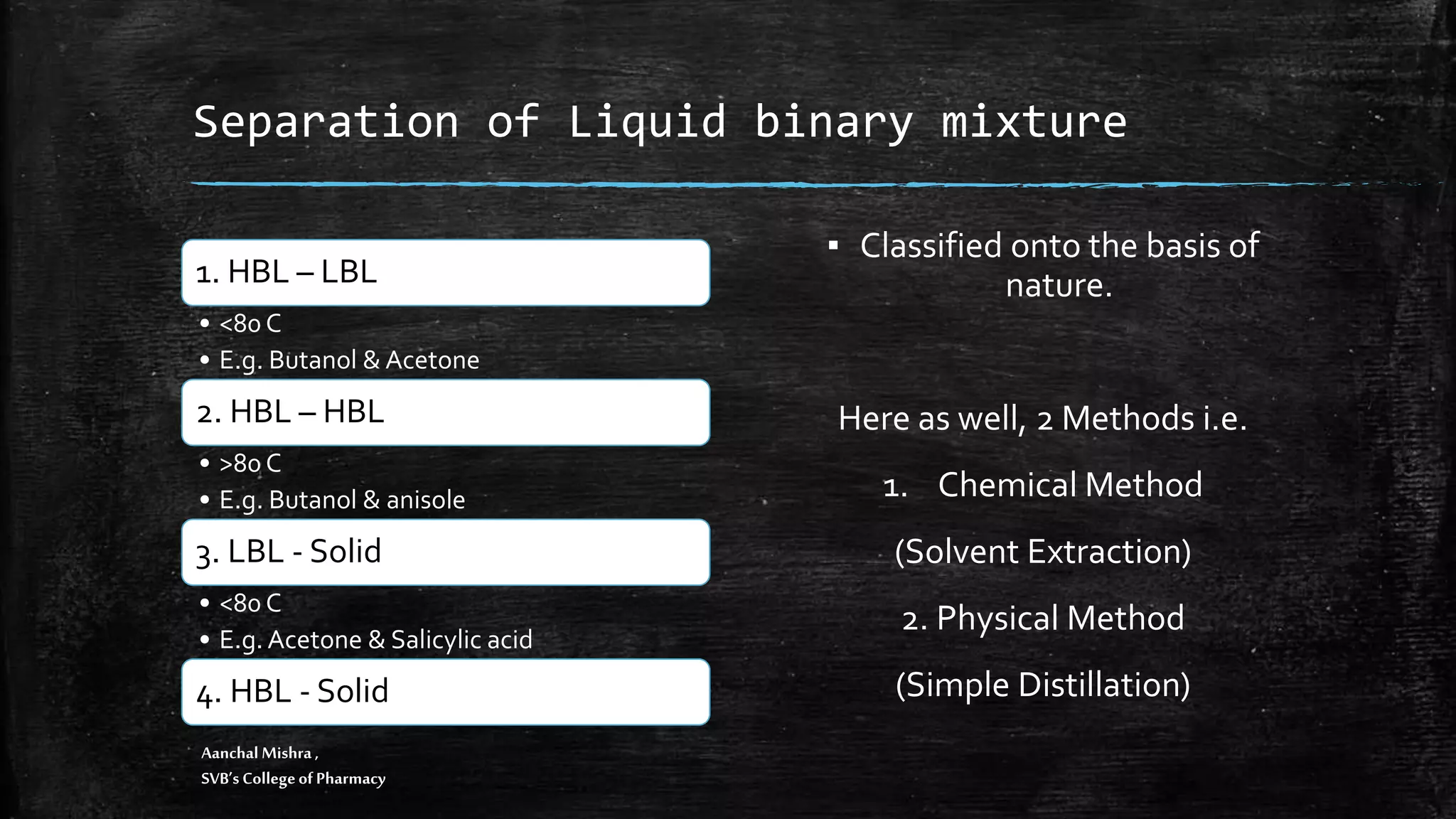
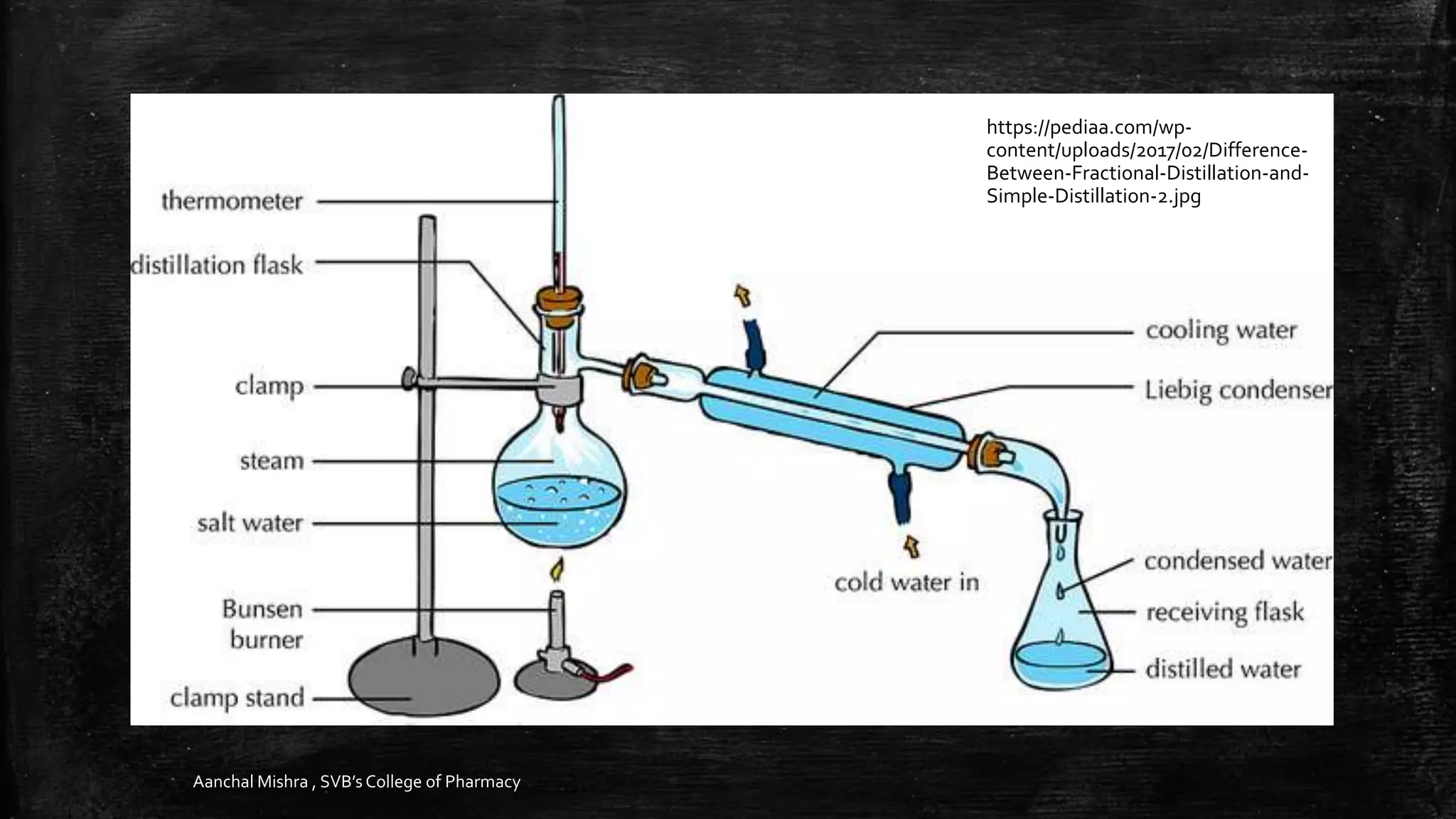
This document discusses various methods for separating binary mixtures, including physical and chemical separation techniques. It covers separating solid-solid, solid-liquid, and liquid-liquid mixtures. Physical separation methods include distillation, crystallization, filtration, and solvent extraction. Chemical separation methods involve the use of reagents like water, sodium bicarbonate, hydrochloric acid, and sodium hydroxide to separate mixtures based on differences in solubility or reactions of the components. Specific examples are provided to illustrate how these separation methods can be applied to separate mixtures like chloroform, urea, benzoic acid, and other compounds.