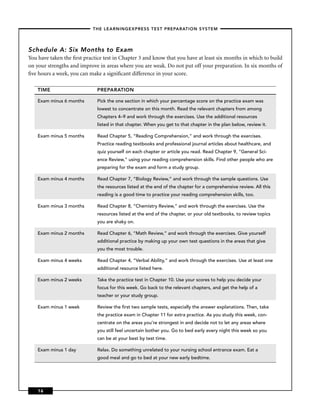
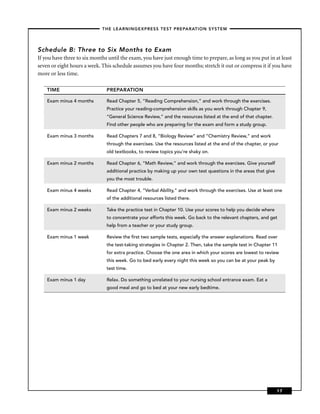
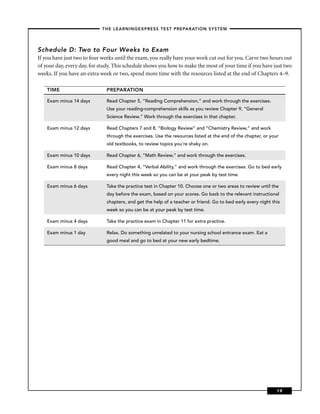
This chapter discusses career opportunities in nursing and preparing for the nursing school entrance exam. There is a high demand for nurses due to an aging population and technological advances. Nurses work in hospitals, doctors' offices, long-term care facilities, patients' homes, and communities. The chapter outlines educational requirements to become a nurse and the importance of this book for preparing for the entrance exam.



![Copyright © 2009 LearningExpress, LLC.
All rights reserved under International and Pan-American Copyright Conventions.
Published in the United States by LearningExpress, LLC, New York.
Library of Congress Cataloging-in-Publication Data
Nursing school entrance exam : your guide to passing the test. — 2nd ed.
p. : cm.
Includes bibliographical references and index.
ISBN-13: 978-1-57685-705-2 (alk. paper)
ISBN-10: 1-57685-705-0 (alk. paper)
1. Nursing schools—United States—Entrance examinations—Study guides. I. LearningExpress (Organization).
[DNLM: 1. Nursing—Examination Questions. WY 18.2 N975905 2009]
RT79.N86 2009
610.73076—dc22
2009021316
Printed in the United States of America
987654321
2nd Edition
ISBN 13: 978-1-57685-705-2
For more information or to place an order, contact LearningExpress at:
2 Rector Street
26th Floor
New York, NY 10006
Or visit us at:
www.learnatest.com](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-4-320.jpg)


































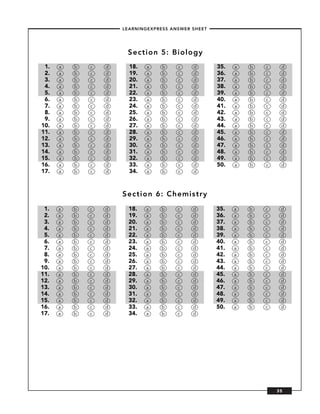































![–PRACTICE EXAM I –
5. Which of the following describes the primary 10. Which of the following represents t-butane?
structure of proteins? a. CH3 – CH2 – CH2 – CH3
a. the collective shape assumed by all of the
b. CH3
chains in a protein containing multiple chains
|
b. the folding of an individual protein molecule
CH3 – C – CH3
c. the regular repeated shape of the protein mol-
|
ecule’s backbone
CH3
d. the sequence of amino acids bonded together
by peptide bonds c. CH3 – CH2 – CH3
23Na
d. H
6. has an atomic number of 11. How many
|
neutrons does it have?
CH3 – C – CH3
a. 11
|
b. 12
CH3
c. 23
d. 34
11. Which electron configuration describes the most
reactive element?
7. The number of protons in an atom is always
a. 1s2 2s2 2p6
equal to its
b. 1s2 2s2 2p5
a. mass number.
c. 1s2 2s2 2p4
b. atomic number.
d. 1s2 2s2 2p3
c. number of isotopes
d. number of neutrons
12. Which of the following is NOT a Lewis base?
a. C6H10O
8. NaOH HCl NaCl + H2O
b. H-O-CH3
The reaction shown here is best described as
c. Na
which of the following?
d. CH3-CH2-CH2-CH(NH2)-CH3
a. base acid salt water
b. metal acid salt hydrogen
13. Which of the following is the correct, balanced
c. metal oxide acid salt water
equation for the combustion of propane?
d. metal carbonate acid salt carbonate
a. C3H8(g) + 5O2(g) + N2(g) → 3CO2(g) + 2NO2(g)
acid (unstable)
+ 4H2(g)
b. C3H8(g) + 502(g) → 3CO2(g) + 4H2O(g)
9. Chlorine, atomic number 17, becomes an ion
c. C3H8(g) + 6O2(g) + 2H2(g) → 3CO2(g) +
when it bonds with Sodium to form salt. How
6H2O(g)
many electrons does that ion have?
d. C3H8(g) + O2(g) + 4H2O(g) → 3CO2(g) + 6H2(g)
a. 0
b. 1
14. What is the electron configuration of a Cl– ion?
c. 17
a. [Ne]3s23p5
d. 18
b. [Ne]Ss2p63d1
c. [Ne]3s23p4
d. [Ne]3s23p6
68](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-76-320.jpg)
















![–PRACTICE EXAM I –
periodic table, will have to gain one electron table, with Cl being rightmost. Cl is also above
to have eight valence electrons. This gives it a Br, so it is the most electronegative atom.
total of 18 electrons. 17. b. This is the definition of the boiling point. At
10. d. A butane is an alkane with four carbon atoms. temperatures higher than the boiling point,
The t in t-butane stands for a tertiary carbon. the vapor pressure of the liquid is greater than
The central carbon of choice d is tertiary external pressure and molecules begin to
because it has three other carbon atoms escape in the gaseous phase.
bonded to it. Choice a is also a butane mole- 18. b. Because the question is written with (II) after
cule, but it is n-butane. Choices b and c are cobalt, the Co ion must be +2. Since the
not butanes. nitride ion is always –3, the formula must be
11. b. The most highly reactive element is fluorine, Co3N2 to have a balanced compound.
1s2 2s2 2p5, which, because of its small size and 19. a. The empirical formula of a compound is the
high electronegativity, will form many com- formula written with the simplest form possi-
pounds, and even oxidize hydrocarbons at ble. C2H6O2 has one molecule of both C and
room temperature. O for every three molecules of H, so the
12. c. A Lewis base is defined as a species that has a empirical formula is CH3O.
nonbonding pair or pairs of electrons that it 20. b. The key to this problem is that 1 ml is equal to
can donate to form new bonds. Sodium is the 1 cm3. Multiplying 110 ml by 0.cm3 g gives
997
only choice that does not have at least one approximately 110 grams.
lone pair of electrons. 21. d. The complete chemical symbol includes two
13. b. Combustion is a reaction in which an alkane numbers. The lower number is the atomic
burns in excess oxygen to give carbon dioxide number, or the number of protons in the
and water. Hydrogen gas, present in all three nucleus. The upper number is the mass num-
incorrect equations, is not a participant in ber, or the sum of the protons and neutrons in
combustion reactions. the nucleus. Therefore, the answer is 16S2–,
33
14. d. The configuration of a chlorine atom in the because there are 18 electrons present.
ground state is [Ne]3s23p5. A Cl– ion has an 22. b. Nonmetal oxides (SO3) and bases (KOH)
additional electron, giving it the same electron react to form salts and water. The solution in
configuration as an argon atom in the ground choice a forms an acid and that in choice c
state, which can also be written as [Ne]3s23p6. forms a salt, but such a reaction would not
15. d. The carbon atom in methane has four sigma give off oxygen.
bonds around it, meaning that it uses its s atomic 23. b. According to the periodic table, the molar
orbital and all three p atomic orbitals to form mass of Al is 26.98, or approximately 27
four sp3 molecular orbitals. The number of grams. Therefore, there are 2 moles (54
atomic orbitals combining always equals the divided by 27) of Al.
number of molecular orbitals formed. 24. d. This problem is simply an equation-balancing
16. c. Electronegativity is a measure of the ability of an problem. The number of molecules of each
atom to attract shared electrons to itself. It element must be the same on each side of the
increases across rows of the periodic table to the equation. Choice d has 8 carbons, 20 hydro-
right and decreases going down columns of the gens, and 26 oxygens on each sicle of the
table. Na, S, and Cl are all in the same row of the equation.
85](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-93-320.jpg)





































































































































![–CHEMISTRY REVIEW–
3. Autoionization of Water Neutralization of acid:
In pure water, 2H2O H3O+ + OH–. HPO4–2 + H+ H2PO4–
Molar concentration of H3O+ = molar concen-
tration of OH–. Neutralization of base:
The ion product of water is Kw; Kw = [H3O+] H2PO4– + OH– HPO4–2 + H2O
[OH–] = 1 10–14. Thus, in pure water: [H3O+] =
[OH–] = 1 10–7 moles/liter. 6. Titration
a. Equivalent
4. pH Equivalent is the gram equivalent weight of any
pH = – log [H+] The pH measures the negative log- base is the amount in grams that can be neutralized
arithm (for presentation of very small numbers in a by 1 mole of H+ ions.
large scale) of the hydrogen ion concentration (in The gram equivalent weight of any acid is the
moles/liter). The pH scale runs from 0 to 14 with amount in grams that can be neutralized by 1 mole
acids in the lower end of the scale (smaller than of OH– ions.
pH 7), whereas bases are at the higher end (greater
than pH 7). b. Normality (N)
Normality is the number of equivalents of the
5. Buffers solute per liter of solution. 1N solution of acid (or
Buffer is a solution of a weak base and its conjugate base) contains 1 equivalent of an acid (or base) per
acid (weak also) that prevents drastic changes in liter of solution.
pH. The weak base reacts with any H+ ions that
could increase acidity, and the weak conjugate acid You Should Review
reacts with OH– ions that may increase the basicity ■ monoprotic, diprotic, and triprotic acids
of the solution. ■ organic and inorganic acids
■ Arrhenius acids and bases
a. Carbonic Acid/Bicarbonate Buffer ■ Bronsted-Lowry acids and bases
Blood pH must be maintained at pH 7.40 by a ■ reactions of acids
buffer system consisting of the couple H2CO3 and ■ activity series of metals
HCO3–. ■ solubilities of salts
■ ionic equations
Neutralization of acid: ■ buffer systems in the body
HCO3– + H+ H2CO3 ■ metabolic acidosis and alkalosis
■ respiratory acidosis and alkalosis
Neutralization of base:
H2CO3 + NaOH NaHCO3 + H2O Questions
65. What is the formula of sulfuric acid?
b. Phosphate Buffer a. HNO3
The principal buffer system inside cells in blood b. H2SO4
consists of the couple [H2PO4– and HPO4–2.] c. HCl
d. H2CO3
222](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-230-320.jpg)
![–CHEMISTRY REVIEW–
66. What is the formula of the hydronium ion? 72. A pH of 4 denotes times fewer
a. H+ than a pH of 3.
b. NH4+ a. 10 . . . hydrogen ions
c. H3O+ b. 4 . . . hydrogen ions
d. H2O+ c. 10 . . . water molecules
d. 20 . . . hydroxide ions
67. The pH of a blood sample is 7.40 at room tem-
perature. The pOH is therefore 73. Which of the following is considered to be neu-
a. 6.60 tral on the pH scale?
b. 7.40 a. pure water
c. 6 10–6 b. pure saliva
d. 4 10–7 c. pure blood
d. pure urine
68. As the concentration of hydrogen ions in a solu-
tion decreases, 74. A substance that functions to prevent rapid,
a. the pH numerically decreases. drastic changes in the pH of a body fluid by
b. the pH numerically increases. changing strong acids and bases into weak acids
c. the product of the concentrations [H+] and bases is called a(n)
[OH–] comes closer to 1 10–14. a. salt.
d. the solution becomes more acidic. b. buffer.
c. enzyme.
69. The pH of an alkaline solution is d. coenzyme.
a. 14.
b. less than 7. 75. Complete the following equation: NaHCO3 +
c. more than 14. HCl NaCl +
d. more than 7. a. HCO3
b. H2CO3
70. A base is a substance that dissociates in water c. HCl
into one or more ions and one or d. H2PO4
more .
a. hydrogen . . . anions Answers
b. hydrogen . . . cations 65. b. The formula is H2SO4.
c. hydroxide . . . anions 66. c. The formula is H3O+.
d. hydroxide . . . cations 67. a. The ion product contrast of H2O is 1 10–14;
–14
[H+] [OH–] = 11 1100–7.40 = 1 10–6.60; pOH =
71. An acid is a substance that dissociates in water 6.60
into one or more ions and one or or
more . pH + pOH = 14.00
a. hydrogen . . . anions pOH = 14.00 – 7.40 = 6.60
b. hydrogen . . . cations 68. b. As the concentration of hydrogen ions
c. hydroxide . . . anions decreases, the pH increases.
d. hydroxide . . . cations
223](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-231-320.jpg)
![–CHEMISTRY REVIEW–
69. d. On the pH scale, 1–7 is acidic, 7 is neutral, Example:
and 7–14 is alkaline. Cl + e– Cl–
70. d. By definition, when a base dissociates in water, Oxidation Number: 0 –1 –1 (Cl is
it produces one or more OH– and one or reduced to Cl–)
more cations. Sum: Na + Cl Na+ + Cl–
71. a. By definition, when an acid dissociates in
water, it produces one or more H+ and one or You Should Review
more anions. ■ redox reactions: cellular respiration, combustion,
72. a. An increase of one pH unit is a tenfold rusting
decrease in hydrogen ions. ■ oxidizing agents
73. a. The pH of pure H2O is 7. [H+] = [OH–] ■ reducing agents
74. b. This is the definition of a buffer.
75. b. Metal bicarbonate + an acid salt + Questions
carbonic acid. 76. The number of electrons lost during oxidation
must always equal the
I. Oxidation-Reduction a. charge of the ion.
b. total change in oxidation number.
1. Oxidation State c. number of electrons gained in the reduction.
Oxidation state (or oxidation number) is the num- d. number of electrons gained by the reducing
ber of charges carried by an ion in an atom, or the agent.
number of charges that an atom would have in a
[neutral] molecule if electrons were transferred com- 77. What is the oxidation number for nitrogen in
pletely. Oxidation numbers enable the identification HNO3?
of oxidized (increase in oxidation number) and a. –2
reduced (reduction in oxidation number) elements. b. +5
The sum of the oxidation numbers of all atoms c. –1
in the formula of a neutral compound is zero (or d. –5
equal to the charge on the ion for a polyatomic ion).
Answers
2. Oxidation-Reduction (Redox) Reactions 76. c. The number of electrons lost during oxida-
Oxidation corresponds to a loss of electrons. tion must always equal the number of elec-
Reduction corresponds to a gain of electrons. trons gained in the reduction.
Redox (reduction-oxidation) reaction involves 77. b. H = +1; O3 = 3 –2 = –6
an electron transfer between the oxidizing (oxidizes +1 + N – 6 = 0
another by accepting its electrons) and the reducing N = +5
(reduces another by donating electrons) agents.
Example:
Na Na+ + e–
Oxidation Number: 0 +1 –1 (Na is
oxidized to Na+)
224](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-232-320.jpg)
















![–GENERAL SCIENCE REVIEW–
23
interval of one degree F. To convert the Avogadro number: 6.0mol10 . In a “mole” of atoms
e
numerical scale of ° F into the numerical scale of any element, for example, there is an Avogadro’s
of ° C, use the equation x° C = 5 (y° F-32). The
9 number of atoms. This number can also be used for
freezing point of water is 0° C or 32° F. the number of molecules of a substance in a chem-
Energy: The joule (J), or calorie (cal); 1 cal = ical mix.
4.184 J. Note that 1 calorie of energy in food Speed of light in a vacuum (c): 3.0 108 m . s
(Cal) is actually a kilocalorie of energy in the Universal gas constant (R): used to relate pres-
metric system. Therefore, 1 Cal = 1,000 cal = sure, temperature, and volume of a gas in the gas law.
1 kcal. Also, power is energy summed up [8.314 Jmol K or 0.08206 L atmmol K]
10–8
over time. Therefore, another term for Stefan-Boltzman constant 5.672 – K4–s J . It is used
m
energy is the kilowatt-hour (kW-h) [or joule to relate the energy of radiation of a material body
second (J.S.)]. (say, the sun) to its surface temperature.
Power: watt (W)
milliwatts (mW) You Should Review
kilowatts (kW) ■ major scientists
■ major experiments and findings
b. Powers of Ten and Constants ■ units of metric system
Powers of ten with prefix names in the metric system: ■ powers of ten
10–12 pico (p), one-trillionth Questions
10–9 nano (n), one-billionth 1. Who wrote The Origin of Species by Means of
10–6 micro (µ), one-millionth Natural Selection, which established the theory of
10–3 milli (m), one-thousandth evolution?
10–2 centi (c), one-hundredth a. Charles Darwin
103 kilo (k), thousand b. William Gilbert
106 mega (M), million c. Aristotle
109 giga (G), billion d. René Descartes
1012 tera (T), trillion
1015 peta (P), quadrillion 2. If you are measuring how water chemistry
changes in a river in the days after a flood, the
Constants: Relating properties in the calcula- time measurement is the
tions of science has resulted in universal constants a. independent variable.
for major laws. These constants are units that work b. independent constant.
out to multiply the other properties in a way that c. dependent variable.
makes the total units equal on both sides of scien- d. dependent constant.
tific equations. You do not have to memorize the
numbers, but you should be familiar with the exis- 3. The prefix tera- refers to which unit in the metric
tence and use of these constants. system?
a. thousand
b. trillion
c. ten thousand
d. three
241](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-249-320.jpg)












































![–GENERAL SCIENCE REVIEW–
All devices, from refrigerators to light bulbs to Nuclear fusion would use energy from fusing
cars, can be quantified in terms of efficiency. hydrogen into helium, fusing the nuclei of atoms
Improvements in energy efficiency can cut down on (which is the process that takes place in the center of
pollutants and the use of fossil fuels, which not only the sun). Fusion requires enormous temperatures
are limited but produce the greenhouse gas carbon and pressures in the fusion reactor’s center, which
dioxide. will probably use incredibly high-tech magnetic
“bottles” to hold the reactants (because nothing
e. Future Energy Technologies material could withstand those conditions). Fusion
Research continues on future energy technologies, has been accomplished in high-energy physics labs,
on sources of energy that do not emit carbon diox- but no fusion energy plants exist yet.
ide and are renewable. Carbon sequestration is a technology that stops
Hydrogen can be burned with oxygen to pro- the emission of CO2 (by trapping and disposing of
duce harmless water (vapor). However, hydrogen carbon dioxide waste) and would allow humans to
does not occur naturally. To have a hydrogen econ- continue burning fossil fuels, depending on supply.
omy in the future, therefore, we need to make One possibility is to pipe carbon dioxide deep into
hydrogen from the splitting of water, which requires the ocean (but this might make conditions intoler-
an energy source, like fossil fuel or solar energy. ably acidic for some benthic marine life). Another
(Hydrogen can also be made from natural gas possibility is to pipe it into deep aquifers of salty,
[methane], but this creates CO2, so to avoid the unusable water far beneath the land surface. But
emission of CO2, it would have to be sequestered, would the CO2 leak back up into the atmosphere? A
see the next column.) small industrial project off the shores of Scandi-
Wind energy uses the pressure of air motions to navia is currently injecting CO2 into the ocean.
turn turbines to make electricity. Many large wind Much remains to be tested with these technologies
turbines are going up all over the world, particularly as well.
in northern Europe. These have blades 100 feet or
more in length. Wind energy is site-specific. In the 4. Systems of Matter and Life
United States, for example, states such as the Dako- The biosphere is an interacting system of matter
tas and the western part of Texas have particular and energy, of humans and nature.
potential for wind development. If set up in farm
fields, only a small percent of the land is used, and a. Waste Disposal
farmers can still grow their crops under the tur- Municipal solid waste describes general garbage.
bines; the land would then do double duty. Disposal methods include landfills, combustion,
Solar energy has two main types: solar thermal recycling, and the composting of organics.
energy that uses sunlight to heat water or air for Sewage describes liquid and solid body wastes
direct use, mainly for domestic water heating or treated in sewage treatment plants. A number of
wintertime home heating; and solar photovoltaic steps are involved: Preliminary and primary treat-
energy that uses solar cells (silicon cells, originally ments remove debris and organic particles, respec-
perfected by NASA for space use) to create electric- tively. Secondary treatment involves bacteria in
ity directly from the photons of the sun. Like wind aqueous slurries. The bacteria consume the dis-
electricity, photovoltaic electricity is increasing, but solved organics in the sewage. Before the treated
not as much because the costs are still quite high. waste water is put back into a natural water system,
286](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-294-320.jpg)







































![–PRACTICE EXAM II –
25. Which of the following are the general products 30. Give the number of valence electrons for a sulfur
of a combustion reaction? atom (S).
a. C(s), O2, and H2 a. 2
b. C(s), H2O, and O2 b. 4
c. CO2 and H2 c. 6
d. CO2 and H2O d. 16
26. Compounds have a set volume but an unset 31. Which of the following is the electron configura-
shape when they are tion of a neutral atom of Ca?
a. solid. a. [Ar] 3s2
b. liquid. b. [Ar] 3d2
c. gas. c. [Ar] 4p2
d. Molecules always behave this way. d. [Ar] 4s2
27. For every three moles of P2O5 produced by the 32. Which of the following bonds is the most polar?
following reaction, how many molecules of P a. Cl2
are required? b. NaCl
4P 5O2 2P2O5 c. F2
a. 6.02 1023 d. HF
b. 1.20 1024
c. 3.01 1023 33. Which of the following is the correct name for
d. 3.61 1024 Li2SO3?
a. lithium sulfite
28. LiOH + HBr LiBr + H2O b. lithium sulfide
How many grams of lithium hydroxide will you c. lithium sulfate
need to add to your reaction to produce exactly d. lithium disulfate
72.6 grams of lithium bromide?
a. 10 34. What is the oxidation number of sodium in the
b. 20 following reaction?
c. 30 Pb(NO3)2(aq) + 2NaI(aq) PbI2(s) + 2NaNO3(aq)
d. 40 a. +1
b. +2
29. Convert 4.12 102 nm to meters. c. –1
a. 4.12 104 m d. –2
b. 4.12 107 m
c. 4.12 10–4 m 35. Carbon dating involves the decay of a carbon-14
d. 4.12 10–7 m isotope with a beta particle. Which of the follow-
ing equations describes this decay?
a. 14 C 13 B + 1 H
6 5
1
14 C
b. 6 14 N + 0
7 –1
c. 14 C 13 B + 0 n
6 5
1
d. 14 N + 0 n 14 C + 1 H
7
1
6
1
329](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-337-320.jpg)

![–PRACTICE EXAM II –
45. Which of the following does NOT have the elec- 47. Write the correct answer, including correct sig-
tron configuration [Ne] 3s2 3p6? nificant figures, for the following calculation:
a. Cl 4.12 10–3 + 9.54 10–5
b. S2– a. 4.22 10–3
c. K+ b. 4.22 10–8
d. Ca2+ c. 1.37 10–8
d. 13.66 10–2
46. Write the Lewis dot structure for ethylene, C2H4.
a. 48. What is the formula for thallium (III) hydroxide?
H H a. TlOH3
/ b. Tl(OH)3
C=C c. Tl3(OH)
/ d. Tl3(OH)3
H H
b. 49. H2PO–4 OH HPO4–2 H2O
H H Part of the blood’s buffer system is shown above.
/ What is the conjugate acid in this system?
C-C a. H2PO–4
/ b. OH
H H c. HPO4–2
d. H2O
c.
H H 50. Balance the following reaction: C3H8 O2
/ CO2 H2O
:C-C: a. C3H8 5O2 3CO2 4H2O
/ b. C3H8 6O2 3CO2 2H2O
H H c. C3H8 6O2 4CO2 3H2O
d. C3H8 4O2 3CO2 4H2O
d.
H H
/
:C=C:
/
H H
331](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-339-320.jpg)












![–PRACTICE EXAM II –
37. b. The alkaline earth metals are in the second Scoring
group; Mg is the only choice from this group.
38. a. The number of protons is the atomic number, After you take your nursing school entrance exam, a
or the lower number; the upper number is the complicated formula will be used to convert your raw
sum of the protons and neutrons. score on each section of the test into a percentile. The
39. d. The Lewis dot structure shows that there are raw score is simply the number you get right on each
two double bonds in the molecule. Therefore, section; wrong answers don’t count against you. A per-
the total bond strength is 799 2 = 1,598. centile is a way of comparing your score with that of
40. c. The only effect of the addition of a catalyst is other test takers; this number indicates what percent of
to increase the rate of reaction. There is no other test takers scored lower than you did on this section.
change in the composition. First, count the number of questions you got
41. a. Allotropes are two different formats of an ele- right in each section, and record them in the following
ment. Ozone and O2 are two different formats blanks:
for the element oxygen.
42. c. Hydrogen bonds greatly increase the boiling Section 1: of 50 questions right
point of a compound. Hydrogen bonds occur Section 2: of 45 questions right
between molecules that have hydrogen as well Section 3: of 50 questions right
as F, O, or N. Section 4: of 50 questions right
43. a. Oxyacids of halogens are named by the num- Section 5: of 50 questions right
ber of oxygens attached. HClO is hypochlor- Section 6: of 50 questions right
ous acid, HClO2 is chlorous acid, HClO3 is
chloric acid, and HClO4 is perchloric acid. Next, convert your raw score into a percentage for
44. c. A decomposition reaction involves a single each section of the exam. (Remember that this per-
molecule breaking down into two separate centage is not the same as a percentile.) By now, your
molecules. quantitative ability should be good enough to tell you
45. a. The electron configuration for Cl is [Ne] how to arrive at a percentage, but if you’ve forgotten,
3s23p5. refer back to the Scoring instructions in Chapter 3.
46. a. Only choice a has all the octets filled and no Now, you can compare your scores on this test
formal charges. Other choices leave impossi- with those on the first practice exam. Chances are, your
ble or unstable structure (choice d), unfilled scores went up. If they didn’t, it’s probably because you
octets (choice b), or formal charges. took the first practice exam without having to worry
47. a. 4.12 10–3 9.54 (10–3 10–2) = (4.12 about time, whereas in this exam, you had some fairly
9.54 10–2) 10–3 = 4.22 10–3 tight time limits to meet.
(two decimal places as in 4.12 and 9.54) So if your scores went down between the first
48. b. The weak base thallium (III) hydroxide has a practice exam and this one, the problem is not so much
formula of Tl(OH)3 which only changes to the limits of your knowledge as your ability to work
TI3+ in a very strong acid. quickly without sacrificing accuracy. In that case,
49. a. The conjugate acid, or proton donor, in the reread Chapter 2, “LearningExpress Test Prep System,”
system shown here is H2PO–4. for tips on how to improve your time management
50. a. This is the only balanced option. during the exam. Then, practice your time manage-
ment skills on the sample exam in the next chapter.
344](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-352-320.jpg)

















































![–PRACTICE EXAM III –
43. d. Nerves are composed of nervous tissue, not 4. a. The molar ratio of Fe2O3 to Fe is 1:2, so the
connective tissue. number of moles Fe produced is twice the
44. b. The renal system, also called the excretory sys- number of moles Fe2O3 used.
tem, consists of the kidneys and excretory 5. b. Magnesium is in group II, so it has two
accessory organs. valence electrons.
45. a. Because this is a recessive trait, to present the 6. d. Oxidation: increase of the oxidation # of N
disorder an individual must be homozygous from NH3 [–3] to N2 [0]. Oxidizing agent:
recessive for the disease. Even with a short- 6NO2 (g); the other reactant, 8NH2 (g) is the
ened life expectancy the gene is not expected reducing agent.
to leave the gene pool, eliminating choice b. 7. b. Oxidation: increase of the oxidation # of Sn
Choice d is not true, because a parent carrying from Sn [0] to SnCl62– [+4]. Oxidizing agent:
one recessive gene will not show symptoms. 4NO3– (aq), while Sn (s) is the reducing agent
Choice d is not true, because carriers are (it is oxidized).
resistant to malaria, which is extremely useful 8. c. Balance Mg first [1 in Mg(s) for 1 in
in parts of the world where malaria is a risk. Mg(OH)2], then O [2 in 2H2O for 2 in
46. c. The junction of two nerve cells is called a Mg(OH)2], and finally H [4 in 2H2O for 2 in
synapse. Mg(OH)2 and 2 in H2].
47. a. Oogenesis is the name of the process in which 9. d. Cr in Cr(NO3)3 is displaced by Al.
the ova (egg cells) are produced and grow in 10. b. Combination of PF3 (g) and F2 (g).
the ovary. Special ovarian cells called oogonia 11. a. Ba(OH)2 (aq) + 2HNO3 (aq) Ba(NO3)2
divide repeatedly to make large numbers of (aq) + 2H2O (l)
prospective eggs called oocytes. The left side of the equation must equal the
48. d. Asexual reproduction occurs when a single right side of the equation for all atoms:
animal alone produces genetically identical 1 Ba [in Ba(OH)2] for 1 Ba [in Ba(NO3)2],
offspring. In vivo fertilization occurs when 2 N (in 2 HNO3) for 2 N [in Ba(NO3)2],
one animal fertilizes another internally. 8 O [2 in Ba(OH)2 and 6 in 2HNO3] for 8 O
49. c. The ossicles, utricle, and cochlea are all com- [6 in Ba(NO3)2 and 2 in 2H2O]
ponents of the human ear. 4 H [2 in Ba(OH)2 and 2 in 2HNO3] for 4 H
50. c. Alcohol acts as a depressant, not as a stimulant. [4 in 2H2O]
12. b. Oxidation: increase of the oxidation # of Si
Section 6: Chemistry from [0] in Si (s) to [+4] in SiCl4 (l) and
1. a. Protons and C6H12O6 have mass but not reduction: decrease of the oxidation # of Cl2
enough to matter in such small quantities as from [0] in Cl2 (g) to [–4] in SiCl4 (l).
20 molecules. Electrons have almost no mass 13. a. C2H4(g) + 3O2(g) ( 2CO2(g) + 2H2O(g)
regardless of how much you have and so the Oxidation: increase of the oxidation # of C
greatest mass is the 0.5 moles of uranium (U). from [–2] in C2H4 (g) to [+4] in CO2 (g) and
2. d. Three half-lives, 39 hours, leave 1 of the
8 reduction: decrease of the oxidation # of O
iodine-123 undecayed: 13.75 grams. A few from [0] in O2(g) to [–4] in CO2 (g).
more hours, a total of about 44, brings it to 14. b. Only NH3 is not ionic and cannot be broken
less than 12 grams. into ions.
3. a. The correct formula for copper (II) oxide 15. d. 3 ions: 2 NO3– and 1 Mg2+: 3 0.25M =
is CuO. 0.75M greater than 0.4M (Al3+ and 3 Cl–),
0.45M (Sr2+ and 2 Br–), 0.4M (Na+ and Br–).
397](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-405-320.jpg)
![–PRACTICE EXAM III –
16. c. In the equation Ba(NO3)2 + Na2SO4 BaSO4 25. b. Mirror-images are two structures that are not
(sol) + 2NaNO3, 1 mole of sodium sulfate pro- superposable (upon rotation/flipping of the
duces 1 mole of the precipitate barium sulfate structure or not). In (a), (e), (f), we have the
[137.3 (Ba) + 32 (S) + 4 16 (4O) = 233.3 g]. same structure: On rotating the second struc-
So, to produce 10.0 g of barium sulfate, only ture (in plane strictly for (a) and (e) since
( 21303.0 ) 1 mol= 0.04 mol of sodium sulfate.
.3 these are Fischer projections and out of plane
17. b. Only NO is not a simple element. for (f)) by 180°, we obtain the first structure:
18. d. NO is the only compound, made of N and O (a), (e), (f) are not constituted by pairs of
atoms. enantiomers or mirror-images. Set (g) is
19. c. Na+ and Cl– labelled (R),(S) for one and (R),(R) for the
20. b. d = m implies that v = m = 217.05g g/mL–1
v d
5
other structure and cannot therefore consti-
262 mL. tute a set of enantiomers (in which absolute
21. d. 5 (72 – 32) = 5 40 = 22.2° C
9 9 configuration shouldn’t be the same for same
22. b. ° C = 5 (° F – 32) so that ° F = 32 + 9 ° C =
9 5 chiral carbon of the structures).
32 + 9 (25)= 77° F
5 (b), (c), (d), (h) are sets of enantiomers or
23. c. 4.50 102 10–9 m= 4.50 10–7 m mirror-images by the same procedure, (h)
24. d. 4.50 102 103 pm= 4.50 105 pm showing (R),(R) and (S),(S) that is character-
istic of enantiomeric pairs.
a. b.
CH3 CH3 CH3 CH3
H OH HO H H OH HO H
H OH HO H HO H H OH
CH3 CH3 CH3 CH3
c. d.
CH2 OH CH2OH CH3 CH3
H OH HO H H OH Br H
H OH HO H Br H H OH
CH3 CH3 CH3 CH3
e. f.
CH3 CH3
CH3 Br
H OH H OH
(R) (R)
HO H HO H Br H 3C
Cl H H Cl
CH3 CH3
g. h.
H H H CH3
CH3 CH3 CH3 H
(R) (R) (R) (S)
(S) (R) (R) (S)
H H 3C H 3C H
CH3 H H CH3
398](https://image.slidesharecdn.com/nursing-exam-practice-book-110502025229-phpapp02/85/Nursing-exam-practice-book-406-320.jpg)









