Nucleic acid therapeutics recent development
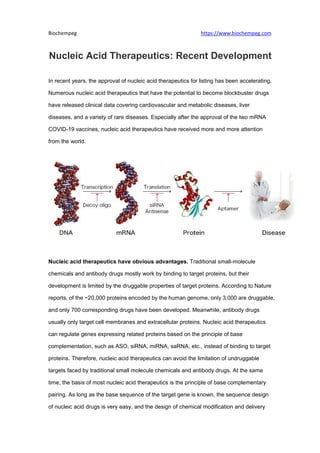
- 1. Biochempeg https://www.biochempeg.com Nucleic Acid Therapeutics: Recent Development In recent years, the approval of nucleic acid therapeutics for listing has been accelerating. Numerous nucleic acid therapeutics that have the potential to become blockbuster drugs have released clinical data covering cardiovascular and metabolic diseases, liver diseases, and a variety of rare diseases. Especially after the approval of the two mRNA COVID-19 vaccines, nucleic acid therapeutics have received more and more attention from the world. Nucleic acid therapeutics have obvious advantages. Traditional small-molecule chemicals and antibody drugs mostly work by binding to target proteins, but their development is limited by the druggable properties of target proteins. According to Nature reports, of the ~20,000 proteins encoded by the human genome, only 3,000 are druggable, and only 700 corresponding drugs have been developed. Meanwhile, antibody drugs usually only target cell membranes and extracellular proteins. Nucleic acid therapeutics can regulate genes expressing related proteins based on the principle of base complementation, such as ASO, siRNA, miRNA, saRNA, etc., instead of binding to target proteins. Therefore, nucleic acid therapeutics can avoid the limitation of undruggable targets faced by traditional small molecule chemicals and antibody drugs. At the same time, the basis of most nucleic acid therapeutics is the principle of base complementary pairing. As long as the base sequence of the target gene is known, the sequence design of nucleic acid drugs is very easy, and the design of chemical modification and delivery
- 2. Biochempeg https://www.biochempeg.com system and sequence design is relatively independent. In contrast, in the discovery and optimization of small molecule and antibody drugs, the optimization of properties such as activity and PKPD requires structural changes, which requires a lot of work. The development process of nucleic acid drugs has not been smooth. Exogenous nucleic acid drugs need to overcome multiple obstacles to enter the body: 1) It is unstable and easily degraded by nucleases in the body; 2) Nucleic acid molecules are immunogenic and will activate the response of the human immune system; 3) The molecular structure is large and negatively charged, which makes it difficult to penetrate the cell membrane; 4) After entering the cell, the nucleic acid molecule needs to escape from the endosome to the cytoplasm (endosome escape). In view of the limitations encountered in the delivery of nucleic acid drugs in vivo, various measures have been applied to try to solve the above-mentioned problems, such as the chemical modification of nucleotides and the application of delivery systems. Chemical modification of nucleotides can improve the stability of nucleic acid molecules and reduce their immunogenicity, including chemical modification of ribose, phosphate backbone, bases, and nucleic acid chain ends. The development of delivery system technology has made it possible to prevent nucleic acid drugs from being degraded by nucleases while improving the efficiency of their entry into cells, such as polymers, lipids (liposomes or LNP), GalNAc, peptides, antibodies, etc. The research and development of nucleic acid drugs are divided into a variety of technical routes, including ASO, siRNA, Aptamer, miRNA, mRNA, saRNA, sgRNA, U1 snRNA, etc.
- 3. Biochempeg https://www.biochempeg.com 1) ASO is a single-stranded structure, chemical modification can effectively improve its stability, immunogenicity, half-life and other properties. Moreover, it is an amphiphilic molecule (hydrophilic and lipophilic), which is relatively less dependent on the delivery system, and can be administered in the form of naked nucleic acid. Curently, there are 7 ASOs are approved by FDA and EMA. 2) siRNA is one of the current research hotspots of nucleic acid drugs. It induces gene silencing through RISC, and the development of delivery system technology has greatly promoted the development of siRNA drugs. At present, 4 siRNA drugs have been approved by the FDA & EMA, and many siRNA drugs that are expected to become blockbuster drugs have published clinical data. 3) Due to the COVID-19 epidemic, the research and development of mRNA vaccines have received more attention and great progress has been made. Two mRNA vaccines have been granted EUA by the FDA. LNP technology is applied in these two vaccines as a delivery system, consisting of a neutral phospholipid, cholesterol, a polyethylene-glycol (PEG)-lipid, and an ionizable cationic lipid, among them, PEG can enhance the stability and lifespan of LNPs. At the same time, protein replacement therapy based on the mRNA route is also being explored. In addition, the research and development of Aptamer, miRNA, saRNA, sgRNA, U1 snRNA and other types of nucleic acid drugs are all proceeding quickly. Product Gene target Indication Administration Approval year ASOs Vitravene, fomivirsen (Ionis Pharmaceuticals) Cytomegalovirus gene (UL123) Cytomegalovirus infection Intravitreal 1998 (withdrawn 2002/2006) ondys 51, eteplirsen (Sarepta Therapeutics) Dystrophin (exon 51) Duchenne muscular dystrophy Intrathecal 2016 Tegsedi, inotersen (Ionis Pharmaceuticals) Transthyretin (TTR) TTR-mediated amyloidosis Subcutaneous 2018 Spinraza, nusinersen (Ionis Pharmaceuticals) Survival of motor neuron 2 (SMN2) Spinal muscular atrophy Intrathecal 2016
- 4. Biochempeg https://www.biochempeg.com Kynamro, mipomersen (Ionis Pharmaceuticals) Apolipoprotein B-100 Hypercholesterolemia Subcutaneous 2013 Waylivra, Volanesoren (Ionis Pharmaceuticals / Akcea) Apolipoprotein CIII Familial chylomicronemia syndrome Subcutaneous 2019 Vyondys 53, golodirsen (Sarepta Therapeutics) Dystrophin (exon 53) Duchenne muscular dystrophy Subcutaneous 2019 (confirmatory tria required) Amondys 45, casimersen (Sarepta Therapeutics) Dystrophin (exon 45) Duchenne muscular dystrophy Subcutaneous 2021 GalNAc−siRNA conjugates Givlaari, Givosiran (Alnylam Pharmaceuticals) ALAS1 Acute hepatic porphyrias Subcutaneous 2019 Leqvio, inclisiran (Novartis/Alnylam Pharmaceuticals) PCSK9 Hypercholesterolemia Subcutaneous 2020 Oxlumo, lumasiran (Alnylam Pharmaceuticals) Glycolate oxidase Primary hyperoxaluria type 1 Subcutaneous 2020 LNP-RNA Onpattro, patisiran (Alnylam Pharmaceuticals) TTR siRNA TTR-mediated amyloidosis Intravenous 2018 Comirnaty, tozinameran (BioNTech/Pfizer) SARS-CoV-2 spike protein mRNA COVID-19 (FDA, emergency use; Switzerland, full approval) Intramuscular 2020 mRNA-1273 (Moderna/NIAID/BARDA) SARS-CoV-2 spike protein mRNA COVID-19 (FDA, emergency use) Intramuscular 2020 AAV vectors Glybera, alipogene tiparvovec (uniQure) Lipoprotein lipase (LPL) (AAV1) LPL deficiency Intramuscular 2012 (withdrawn 2017) Luxturna, voretigene neparvovec-rzyl (Spark Therapeutics) RPE65 (AAV2) Leber congenital amaurosis Subretinal 2017 Zolgensma, onasemnogene beparvovec (AveXis/Novartis) SMN1 (AAV9) Spinal muscular atrophy Intravenous 2019 Adenovirus (Ad) vectors axzevria, AZD1222, ChAdOx1 nCoV-19 (AstraZeneca) SARS-CoV-2 spike protein DNA (ChAdOx1) COVID-19 (FDA and EMA emergency use) Intramuscular 2021 Ad26.COV2.S (Johnson & Johnson) SARS-CoV-2 spike protein DNA (Ad26) COVID-19 (FDA and EMA emergency use) Intramuscular 2021
- 5. Biochempeg https://www.biochempeg.com Convidecia, Ad5-nCoV (CanSinoBIO) SARS-CoV-2 spike protein DNA (Ad5) COVID-19 (Approved in China) Intramuscular 2021 Nucleic acid therapeutics approved by the FDA and EMA. Source: Reference [1] With the advancement of clinical practice and the maturity of related technologies, the approval of nucleic acid drugs has accelerated significantly in recent years. At present, many different kinds of nucleic acid drugs are entering or have been in different clinical stages, and their indications are becoming more extensive and even curing some diseases. It is expected that as more difficulties are overcome, more nucleic acid drugs will be clinically applied. Nucleic acid drugs are expected to become the third-largest type of drugs after small molecule chemicals and antibody drugs. As a global partner, Biochempeg can supply commercial quantities of high-quality functionalized PEGs, which are essential for your PEGylated nucleic acid therapeutics. We will PEGylate your nucleic acid therapeutics and deliver your PEGylated product with a certificate of analysis, as a regular end-product, for further testing at your site. References: [1] Kulkarni, J.A., Witzigmann, D., Thomson, S.B. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021). https://doi.org/10.1038/s41565-021-00898-0 Related Articles: Therapeutic Oligonucleotides on the Rise Understand the COVID-19 mRNA Vaccine PEGylated Drug Delivery Systems for siRNA Drug Development in Cancer Therapy Advantages and Disadvantages of PEGylated Liposomes in Drug Delivery System