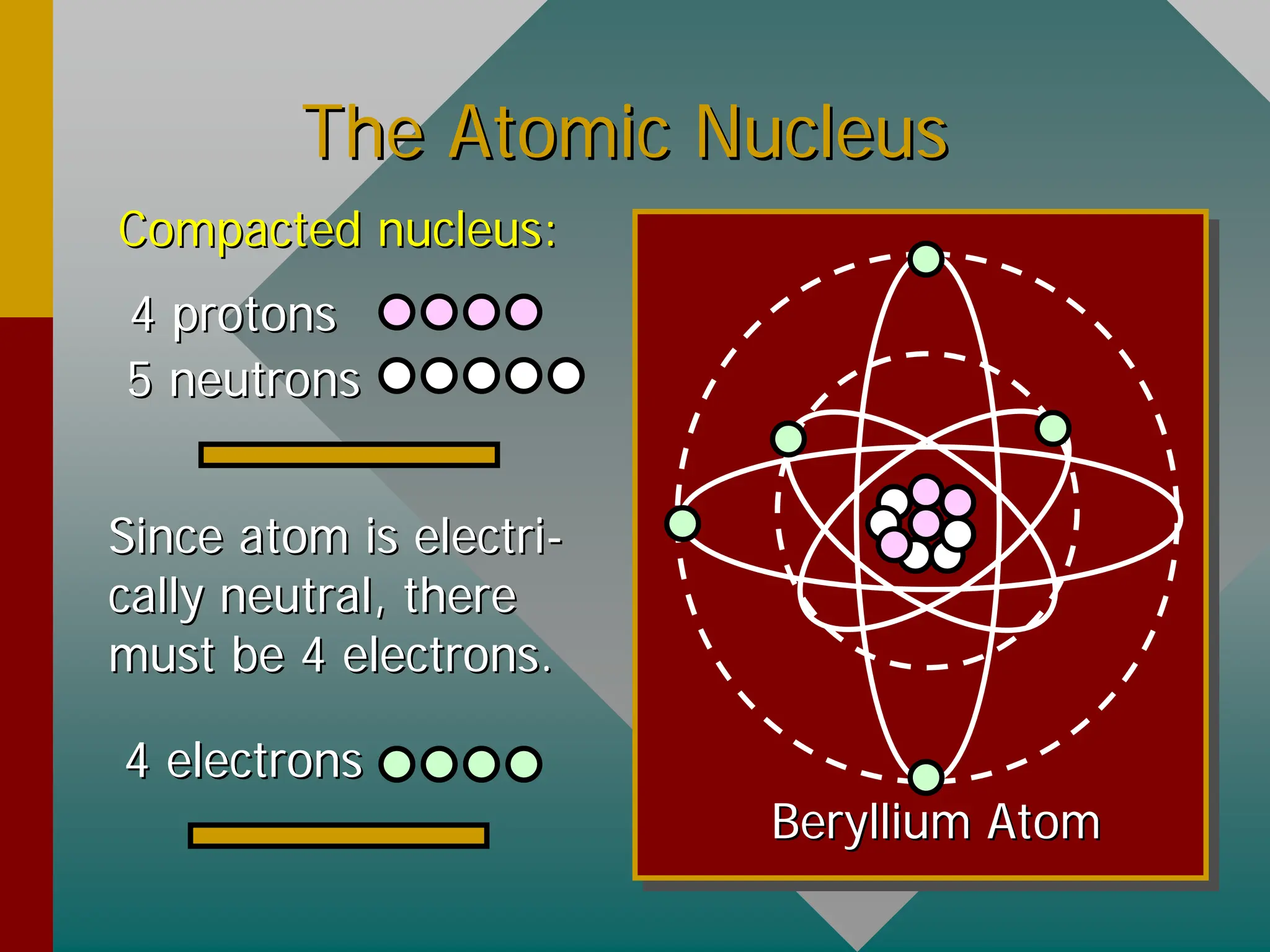
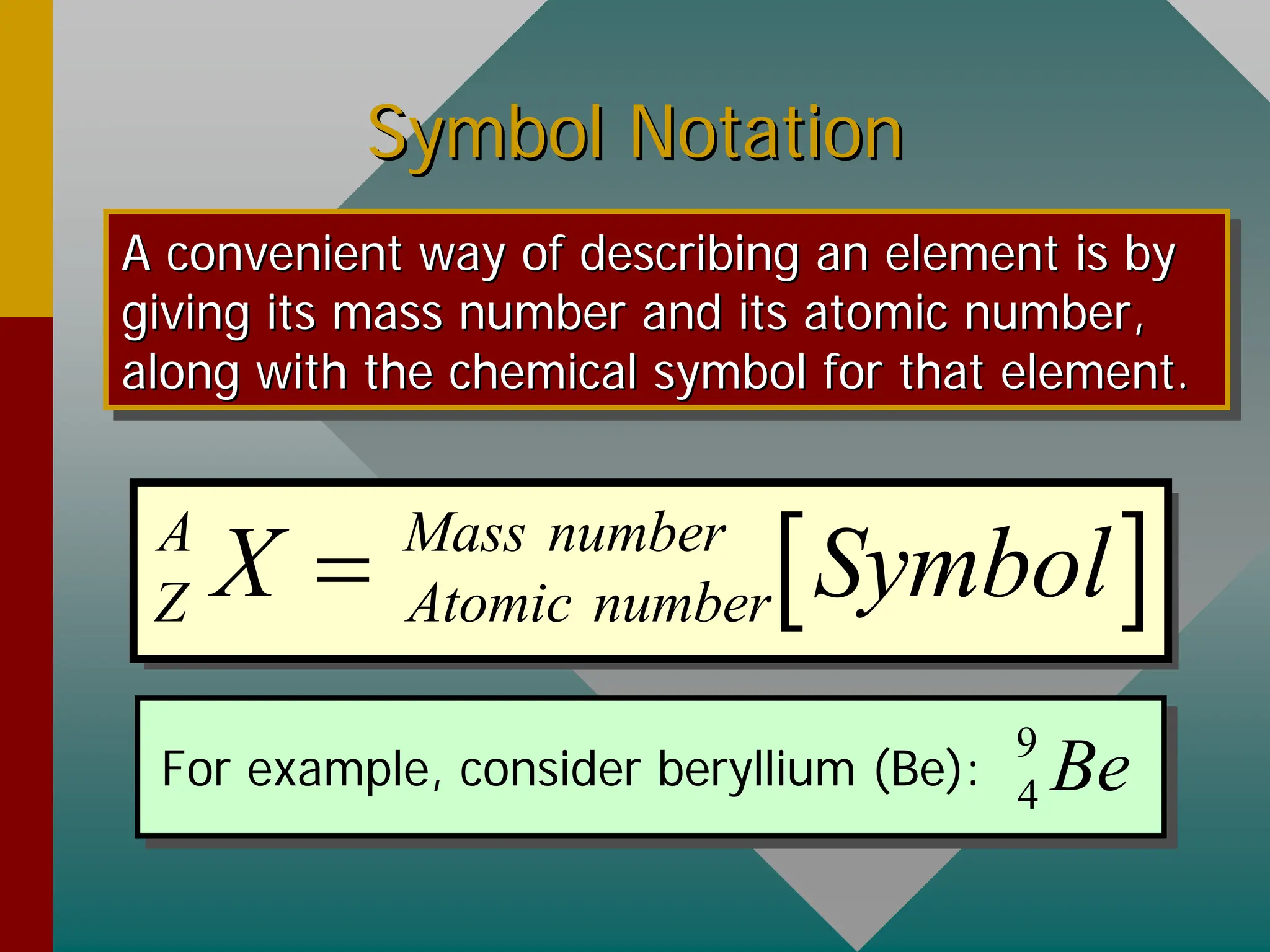
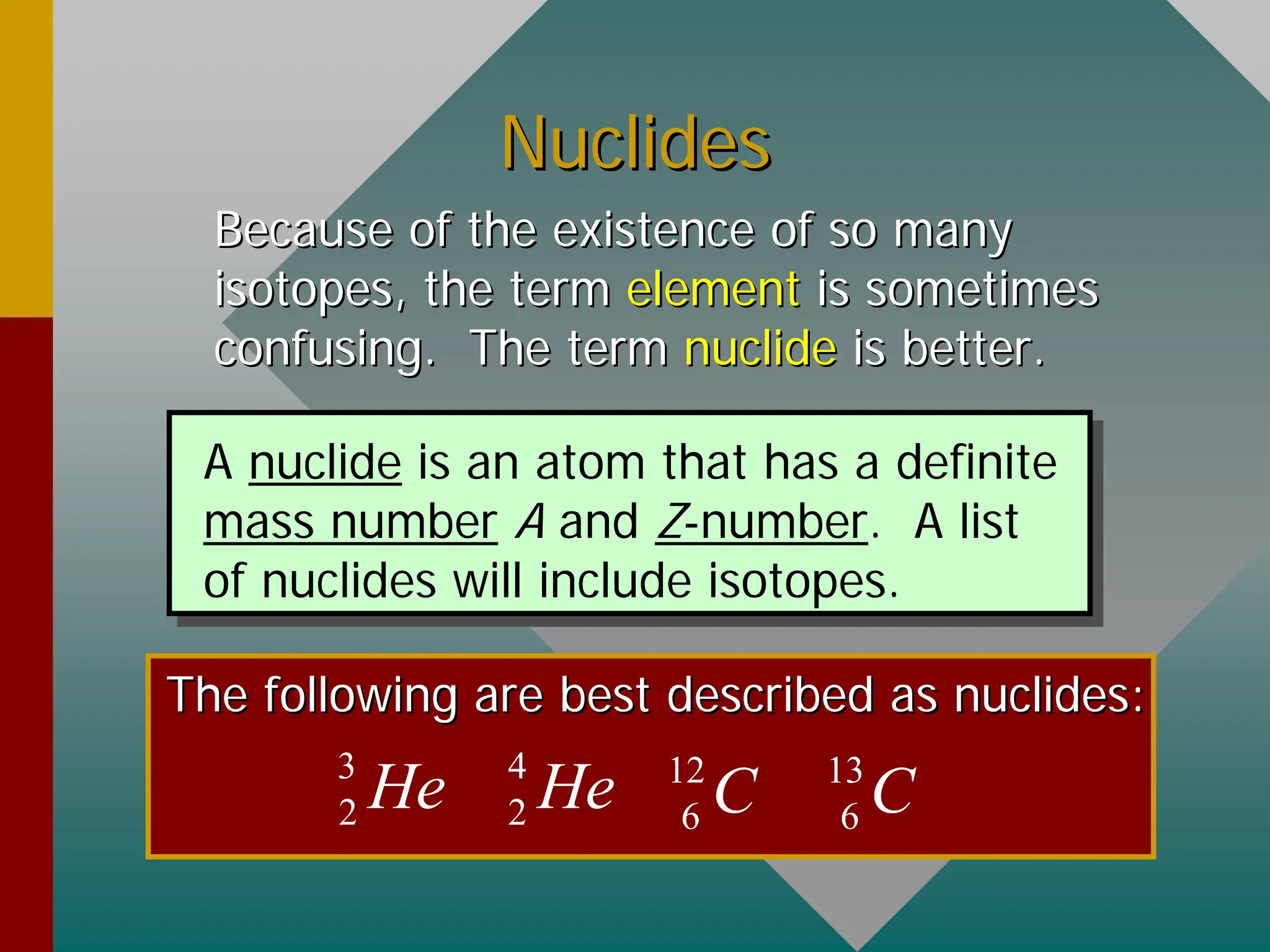
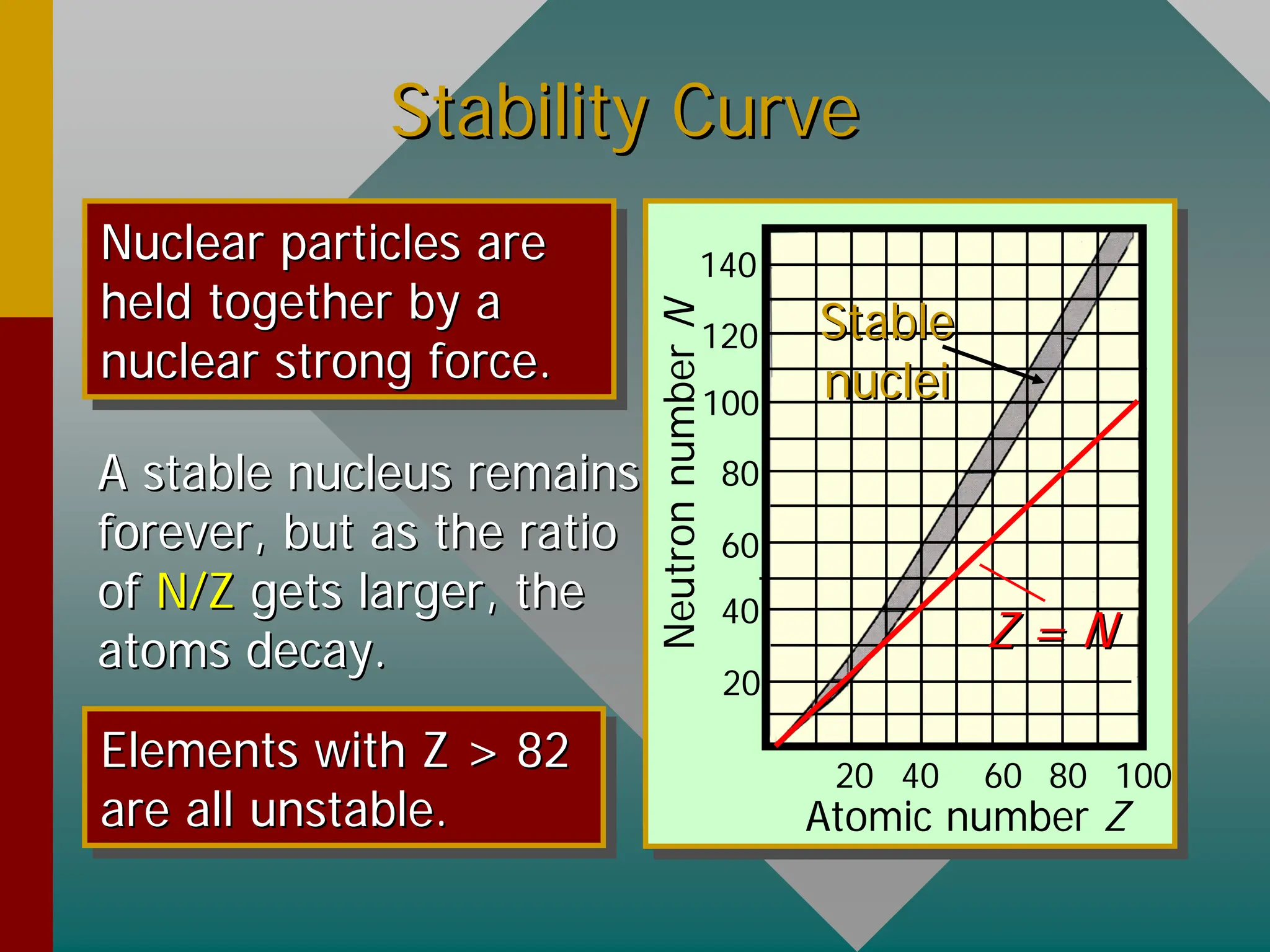
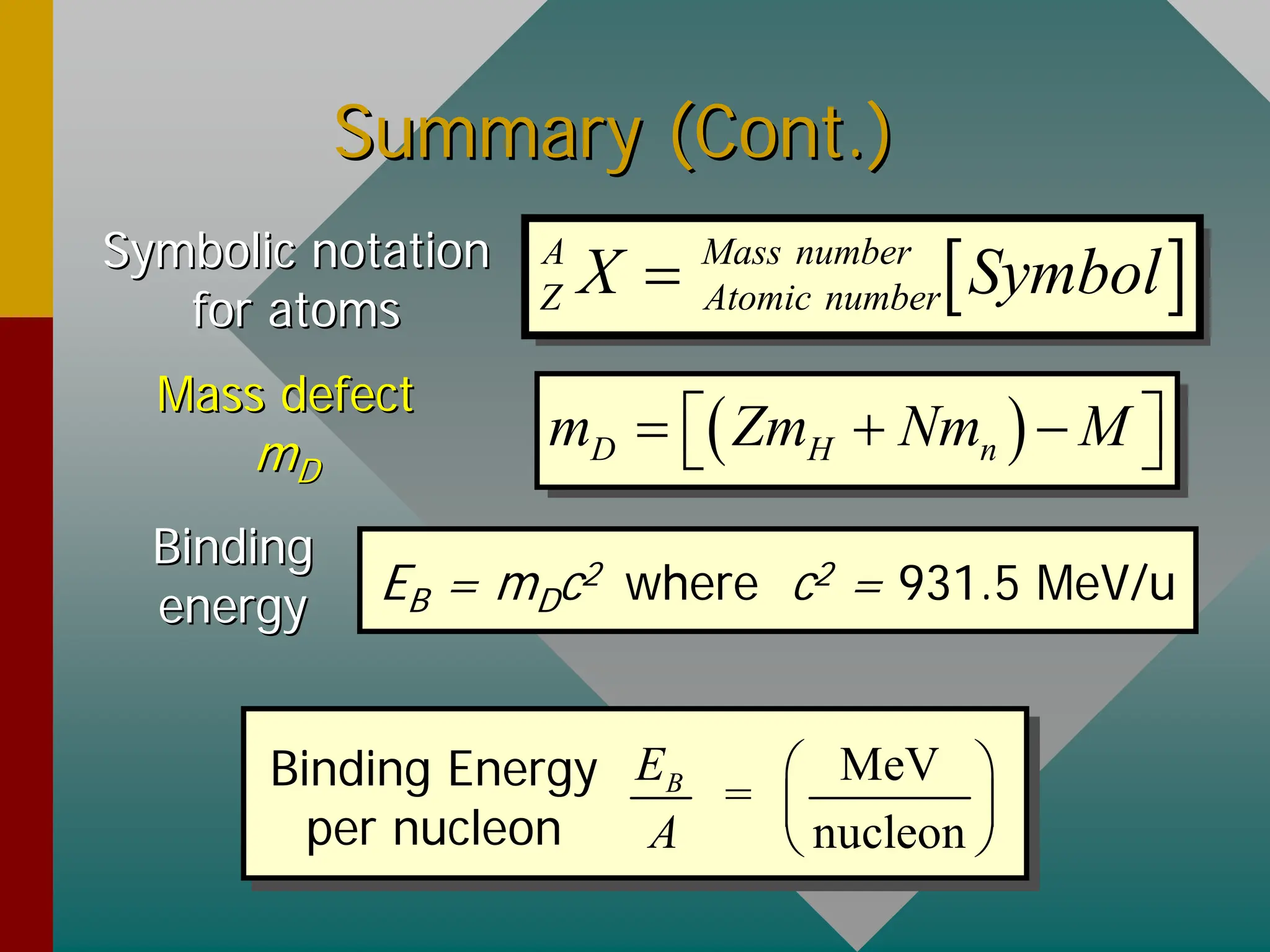
This document provides an overview of nuclear physics concepts. It defines key terms like atomic number, mass number, isotopes, and nuclides. It explains that the nucleus is much smaller than the atom but contains most of its mass. Nuclear reactions and radioactive decay are introduced. Binding energy is defined as the energy required to split a nucleus into protons and neutrons, and binding energy per nucleon is used to compare nuclear stability between elements.