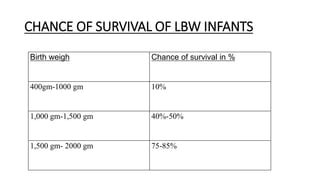
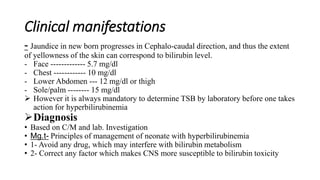
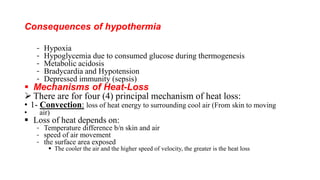
The document provides guidance on caring for newborn babies. It discusses maintaining the airway, cleaning the eyes and skin, umbilical cord care, feeding, and measuring APGAR scores. Care for low birth weight or premature babies is also outlined, including their increased risk of respiratory, temperature regulation, and infection issues due to anatomical and physiological immaturity. Signs of post-term infants and characteristics of premature babies are also summarized.