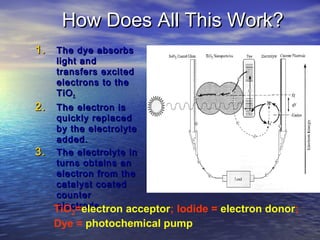
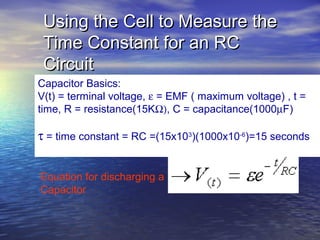
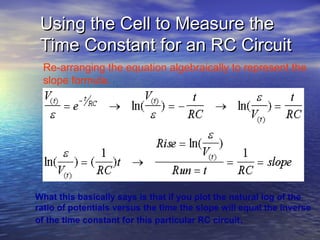
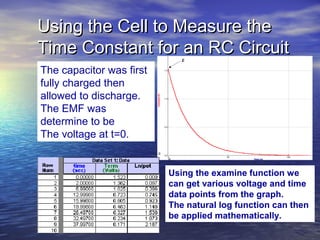
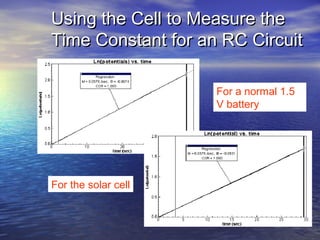
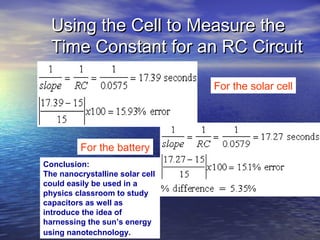
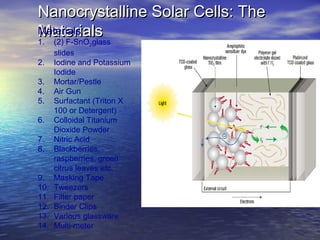
This document outlines a nanotechnology curriculum for high school students presented by Kenneth Bowles. It discusses how nanotechnology concepts can address various science teaching standards and provides an example experiment on constructing nanocrystalline solar cells. The curriculum includes modules on measurement, surface area, electrical applications, reading assignments, and a 15-week science ethics forum. Planned hands-on activities exploring self-assembly and nanofilters are described. In closing, thanks are given to collaborators who provided funding and support for the nanotechnology education program.








![Preparation of Nanotitanium and
Electrolyte Solution
Nanotitanium
1. Add 2-ml of 2,4 – Pentanedione (C 5 H 8 O 2 ) to 100-ml of
anhydrous isopropanol [ (CH 3 ) 2 CHOH ] and stir covered
for 20 minutes.
2. Add 6.04-ml of titanium isopropoxide (Ti[(CH 3 ) 2 CHO] 4
to the solution and stir for at least 2 hours.
3. Add 2.88-ml of distilled water and stir for another 2
hours.
4. The solution must then age for 12 hours at room
temperature.
5. Since you now have a collodial suspension, the
solvent must be evaporated off in an oven to collect
the powder.
Electrolyte solution
1. Measure out 10-ml of ethylene glycol
2. Weigh out 0.127-g of I 2 and add it to the ethylene
glycol and stir.
3. Weigh out 0.83 g of KI and add it to the same
ethylene glycol.](https://image.slidesharecdn.com/nanotechnology-130103072523-phpapp01/85/Nanotechnology-10-320.jpg)