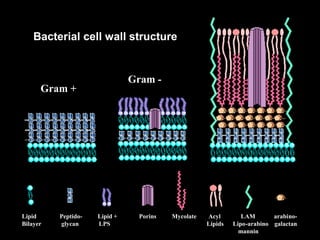
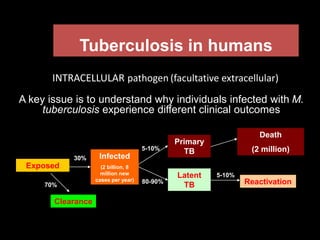
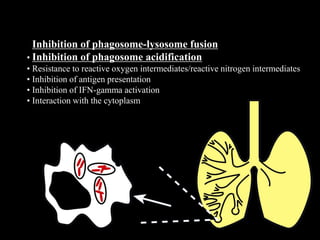
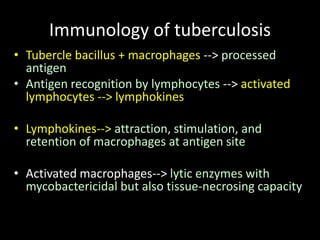
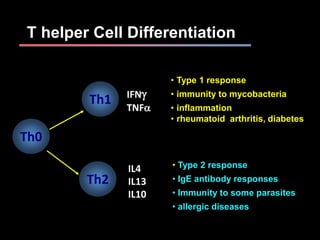
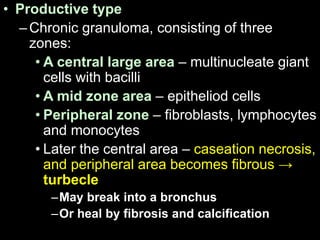
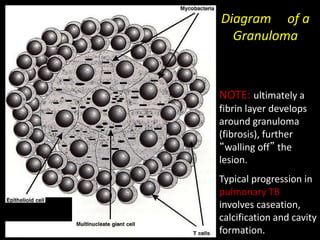
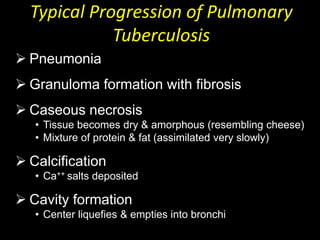
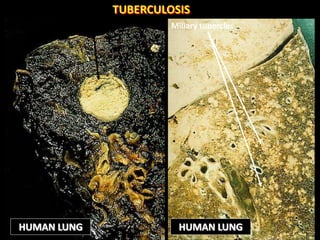
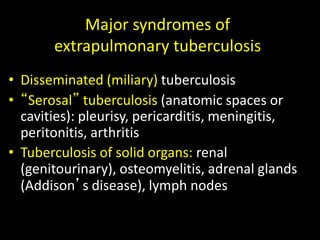
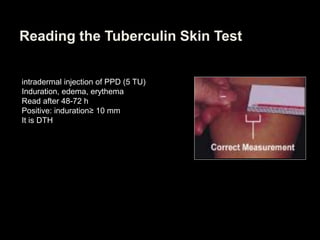
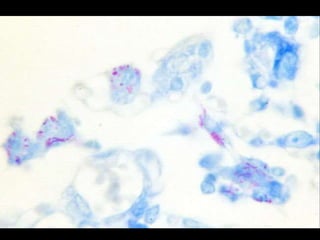
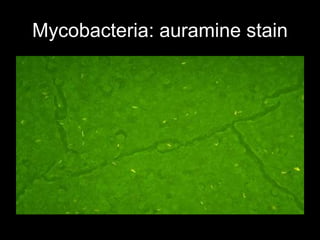
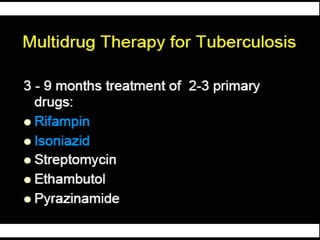
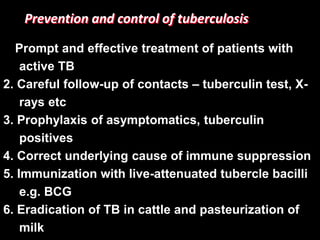
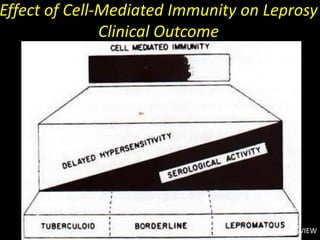
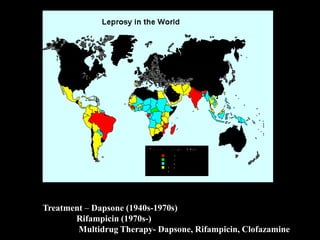
This document provides an overview of mycobacteria, including those of medical importance. It discusses the characteristics of the Mycobacterium genus and important human pathogens such as M. tuberculosis, M. leprae, and M. avium-intracellulare Complex. The pathogenesis and clinical manifestations of tuberculosis are summarized, including the formation of granulomas and typical progression within the lungs. Methods for diagnosis and prevention/control of tuberculosis are also outlined.