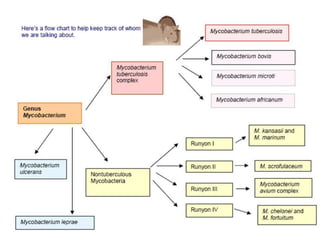
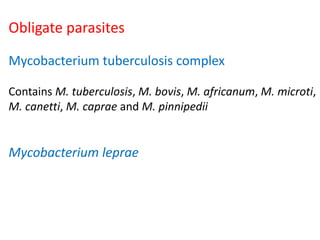
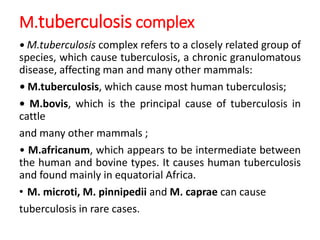
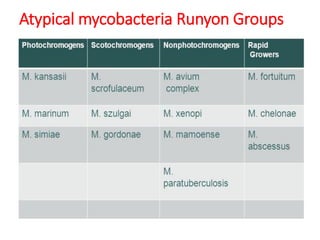
Mycobacterium tuberculosis
Mycobacterium tuberculosis (M. tuberculosis) is a pathogenic bacterium that causes tuberculosis (TB), a serious infectious disease primarily affecting the lungs.
Characteristics
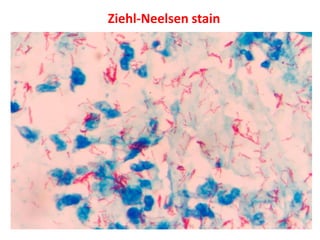
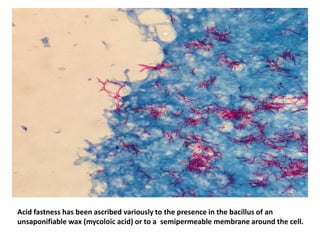
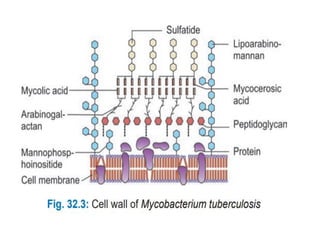
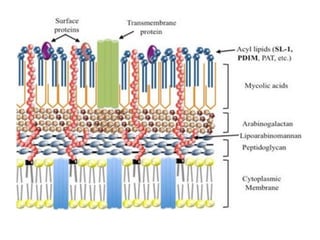
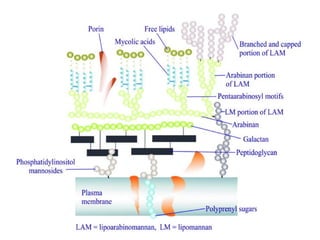
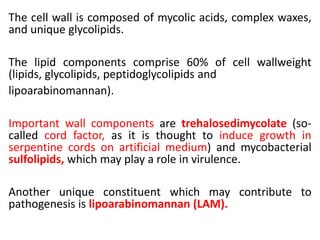
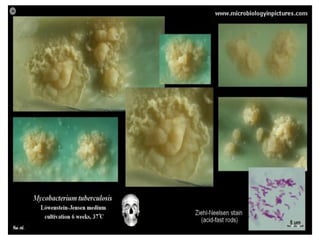
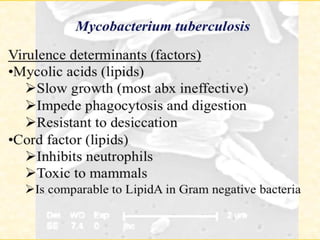
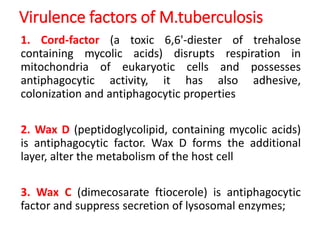
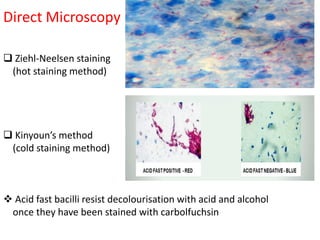
1. *Acid-fast*: M. tuberculosis is an acid-fast bacillus, meaning it retains certain stains even when treated with acid.
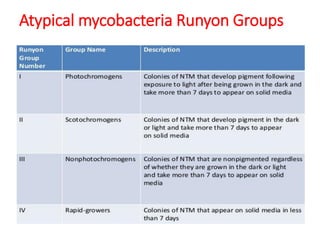
2. *Slow-growing*: M. tuberculosis has a slow growth rate, which can make diagnosis and treatment challenging.
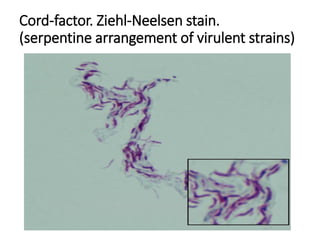
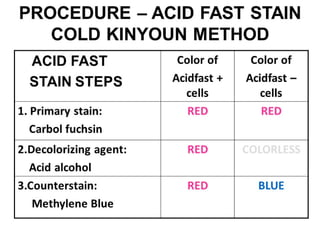
3. *Ziehl-Neelsen stain*: M. tuberculosis can be identified using the Ziehl-Neelsen stain, a special staining technique.
Transmission
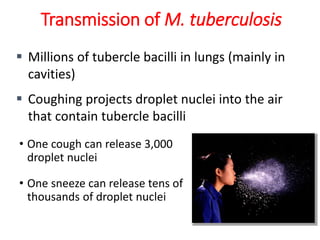
1. *Airborne transmission*: M. tuberculosis is primarily spread through the air when an infected person coughs, sneezes, or talks.
2. *Close contact*: People in close contact with an infected individual are at higher risk of transmission.
Pathogenesis
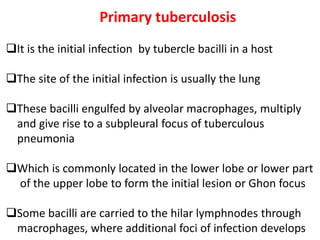
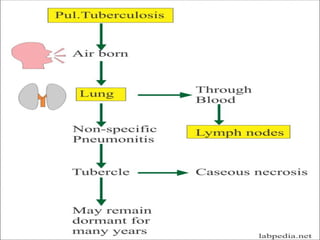
1. *Inhalation*: M. tuberculosis is inhaled into the lungs, where it infects alveolar macrophages.
2. *Intracellular survival*: The bacteria can survive inside macrophages, evading the host's immune response.
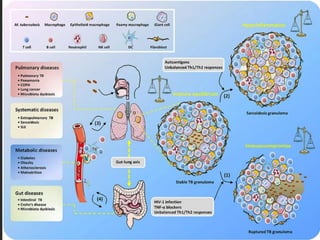
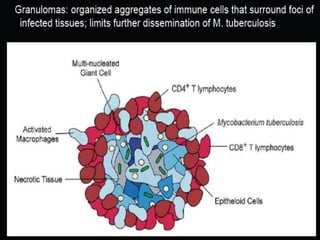
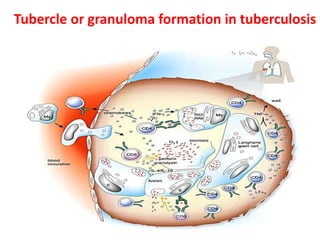
3. *Granuloma formation*: The immune system responds by forming granulomas, which are clusters of immune cells that attempt to contain the infection.
Clinical manifestations
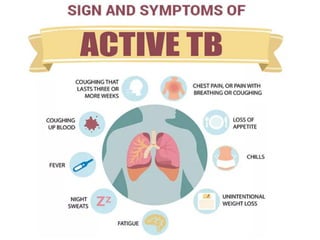
1. *Pulmonary TB*: Symptoms include cough, chest pain, coughing up blood, and difficulty breathing.
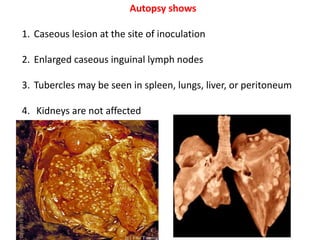
2. *Extrapulmonary TB*: TB can affect other parts of the body, such as the kidneys, spine, or brain.
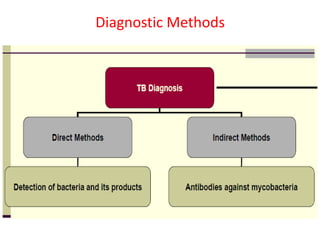
Diagnosis
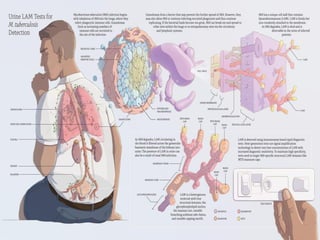
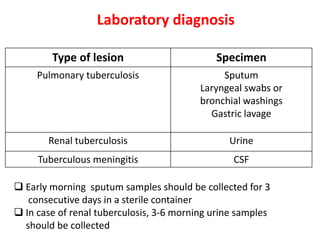
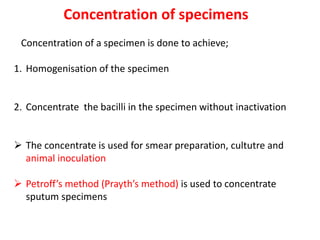
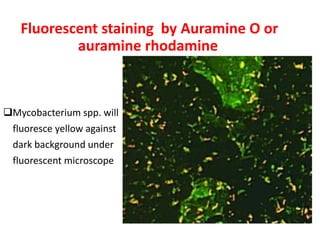
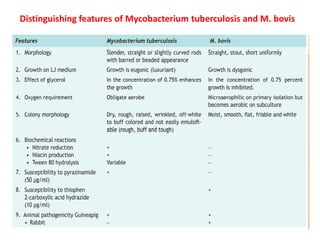
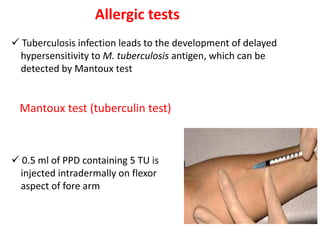
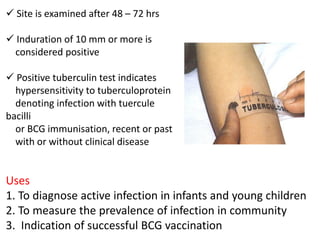
1. *Sputum smear microscopy*: Examination of sputum samples for acid-fast bacilli.
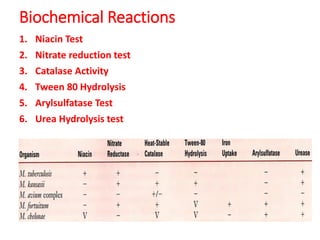
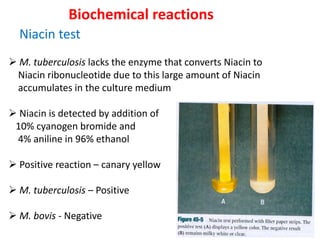
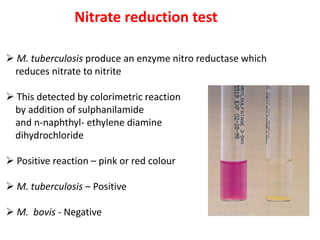
2. *Culture*: Growing M. tuberculosis in culture is a definitive diagnostic method.
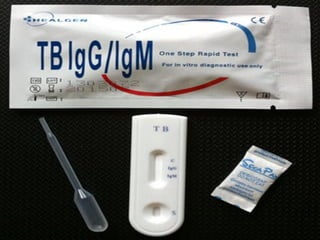
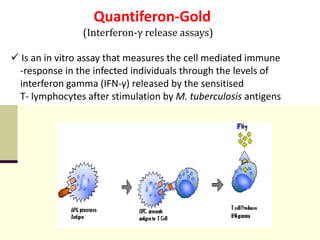
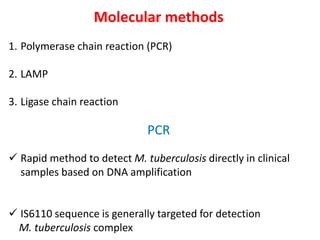
3. *Molecular tests*: PCR (polymerase chain reaction) and other molecular tests can rapidly detect M. tuberculosis DNA.
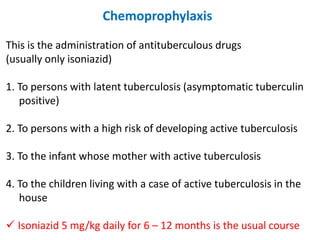
Treatment
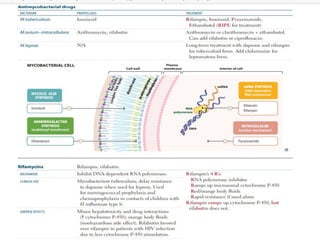
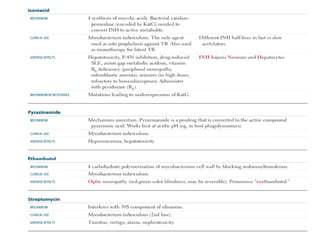
1. *Multidrug therapy*: A combination of antibiotics, typically isoniazid, rifampicin, pyrazinamide, and ethambutol, is used to treat TB.
2. *Long treatment duration*: Treatment typically lasts for 6 months or longer, depending on the case.
Challenges
1. *Drug resistance*: The emergence of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) poses significant challenges.
2. *Global burden*: TB remains a major public health problem worldwide, particularly in low- and middle-income countries.
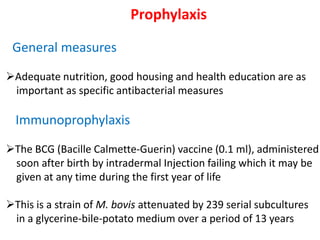
Prevention
1. *BCG vaccine*: The Bacillus Calmette-Guérin (BCG) vaccine provides some protection against severe forms of TB, particularly in children.
2. *Infection control*: Measures such as ventilation, masks, and isolation can help prevent transmission.
Public Health Measures
1. *Surveillance*: Monitoring and tracking TB cases.
2. *Contact tracing*: Identifying and screening individuals who have been in contact with someone with TB.
3. *Education*: Public awareness campaigns on TB prevention and treatment.
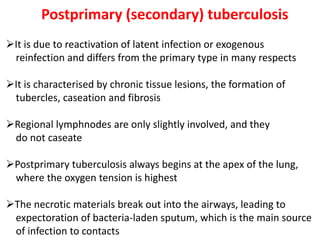
M. tuberculosis is a complex pathogen that requires a comprehensive approach to control and eliminate TB. Ongoing research and development of new diagnostics, treatments, and vaccines are crucial to combating this disease.